CD152 (CTLA-4) determines the unequal resistance of Th1 and Th2 cells against activation-induced cell death by a mechanism requiring PI3 kinase function
- PMID: 15007096
- PMCID: PMC2212725
- DOI: 10.1084/jem.20031058
CD152 (CTLA-4) determines the unequal resistance of Th1 and Th2 cells against activation-induced cell death by a mechanism requiring PI3 kinase function
Abstract
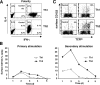
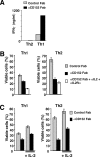
Survival of antigen-experienced T cells is essential for the generation of adaptive immune responses. Here, we show that the genetic and antibody-mediated inactivation of CD152 (cytotoxic T lymphocyte antigen 4) in T helper (Th) effector cells reduced the frequency of nonapoptotic cells in a completely Fas/Fas ligand (FasL)-dependent manner. CD152 cross-linking together with stimulation of CD3 and CD28 on activated Th2 cells prevented activation-induced cell death (AICD) as a result of reduced Fas and FasL expression. Apoptosis protection conferred by CD152 correlated with the up-regulation of Bcl-2 and was mediated by phosphatidylinositol 3 kinase, which prevented FasL expression through the inhibitory phosphorylation of Forkhead transcription factor FKHRL1. We show that signals induced by CD152 act directly on activated T lymphocytes and, due to its differential surface expression on activated Th1 and Th2 cells, induce resistance to AICD mainly in Th2 cells.
Figures

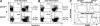




References
-
- Watanabe-Fukunaga, R., C.I. Brannan, N.G. Copeland, N.A. Jenkins, and S. Nagata. 1992. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 356:314–317. - PubMed
-
- Lynch, D.H., M.L. Watson, M.R. Alderson, P.R. Baum, R.E. Miller, T. Tough, M. Gibson, T. Davissmith, C.A. Smith, K. Hunter, et al. 1994. The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity. 1:131–136. - PubMed
-
- Lenardo, M., K.M. Chan, F. Hornung, H. McFarland, R. Siegel, J. Wang, and L. Zheng. 1999. Mature T lymphocyte apoptosis-immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 17:221–253. - PubMed
-
- Pinkoski, M.J., and D.R. Green. 2002. Lymphocyte apoptosis: refining the paths to perdition. Curr. Opin. Hematol. 9:43–49. - PubMed
-
- Datta, S.R., A. Brunet, and M.E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

