Beta 2-Glycoprotein I binds factor XI and inhibits its activation by thrombin and factor XIIa: loss of inhibition by clipped beta 2-glycoprotein I
- PMID: 15007174
- PMCID: PMC374348
- DOI: 10.1073/pnas.0400281101
Beta 2-Glycoprotein I binds factor XI and inhibits its activation by thrombin and factor XIIa: loss of inhibition by clipped beta 2-glycoprotein I
Abstract
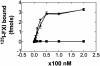
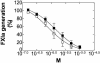
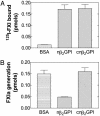
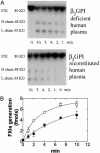
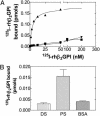
Activation of factor XI (FXI) by thrombin in vivo plays a role in coagulation by providing an important positive feedback mechanism for additional thrombin generation. FXI is activated in vitro by thrombin, or FXIIa in the presence of dextran sulfate. In this report, we investigated the effect of beta(2)-glycoprotein I (beta(2)GPI) on the activation of FXI. beta(2)GPI bound FXI in vitro and inhibited its activation to FXIa by thrombin and FXIIa. The affinity of the interaction between beta(2)GPI and FXI was equivalent to the interaction between FXI and high molecular weight kininogen. Inhibition of FXI activation occurred with lower concentrations of beta(2)GPI than found in human plasma. Proteolytic clipping of beta(2)GPI by plasmin abolished its inhibition of FXI activation. The results suggest a mechanism of regulation whereby physiological concentrations of beta(2)GPI may attenuate thrombin generation in vivo by inhibition of FXI activation. Plasmin cleavage of beta(2)GPI provides a negative feedback that counteracts its inhibition of FXI activation.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

