The effect of eosinophils on collagen gel contraction and implications for tissue remodelling
- PMID: 15008974
- PMCID: PMC1808957
- DOI: 10.1111/j.1365-2249.2004.02396.x
The effect of eosinophils on collagen gel contraction and implications for tissue remodelling
Abstract
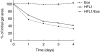
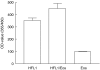
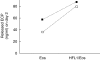
Asthma is characterized by an eosinophilic inflammation and a subepithelial fibrosis in the airways. Eosinophils contain several cytotoxic substances, such as eosinophil cationic protein (ECP), which can promote inflammation and cause tissue damage. This has generated the hypothesis that eosinophils may drive remodelling of extracellular matrix (ECM). To investigate the role of eosinophils we used an in vitro model for remodelling, the three-dimensional collagen gel contraction assay. Two sources of eosinophils were used in this study, isolated human peripheral eosinophils (purity > 95%) and stimulated [interleukin (IL)-5, IL-3 and granulocyte macrophage-colony stimulating factor (GM-CSF)] HL-60 clone 15 cells. Human eosinophils or HL-60 cells were cast together with human lung fibroblasts (HFL1) in type I collagen gels. Both types of eosinophils augmented fibroblast-mediated collagen gel contraction in a time and concentration-dependent manner. At 48 h, the gel area in HFL1/eosinophil co-culture was 46.5% +/- 0.5 (mean +/- s.e.m.) of initial area and in HFL1 culture 52.3% +/- 0.1 (P < 0.001). Respective figures for HFL1/stimulated HL-60 co-culture and HFL1 culture only were 44.1% +/- 0.5 and 52.4% +/- 0.4 (P < 0.001). The release of ECP was increased when fibroblasts were cultured with eosinophils compared to eosinophils cultured alone. In addition, native ECP added to fibroblast gel cultures also augmented contraction. Our results suggest that eosinophils may interact with mesenchymal cells, promoting remodelling of ECM and that ECP constitutes one potential eosinophil-derived mediator driving this process. We conclude that this may be one important mechanism by which eosinophil-ECM interactions will lead to airway tissue remodelling in asthma.
Figures



Similar articles
-
Eosinophil cationic protein stimulates TGF-beta1 release by human lung fibroblasts in vitro.Inflammation. 2007 Oct;30(5):153-60. doi: 10.1007/s10753-007-9032-4. Inflammation. 2007. PMID: 17587163
-
Eosinophil cationic protein stimulates migration of human lung fibroblasts in vitro.Scand J Immunol. 2009 Apr;69(4):381-6. doi: 10.1111/j.1365-3083.2009.02233.x. Scand J Immunol. 2009. PMID: 19284504
-
Effects of human lung fibroblasts on eosinophil degranulation.Allergy. 2000 Dec;55(12):1170-8. doi: 10.1034/j.1398-9995.2000.00623.x. Allergy. 2000. PMID: 11117275
-
Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens.J Leukoc Biol. 2001 Nov;70(5):691-8. J Leukoc Biol. 2001. PMID: 11698487 Review.
-
Eosinophils and eosinophil products in asthma.J Ayub Med Coll Abbottabad. 2002 Oct-Dec;14(4):49-55. J Ayub Med Coll Abbottabad. 2002. PMID: 12688104 Review.
Cited by
-
Eosinophil granulocytes are activated during the remission phase of ulcerative colitis.Gut. 2005 Dec;54(12):1714-20. doi: 10.1136/gut.2005.066423. Epub 2005 May 10. Gut. 2005. PMID: 15886302 Free PMC article.
-
Esophageal dysmotility in patients who have eosinophilic esophagitis.Gastrointest Endosc Clin N Am. 2008 Jan;18(1):73-89; ix. doi: 10.1016/j.giec.2007.09.006. Gastrointest Endosc Clin N Am. 2008. PMID: 18061103 Free PMC article. Review.
-
The effect of PM10 on human lung fibroblasts.Toxicol Ind Health. 2009 Mar;25(2):111-20. doi: 10.1177/0748233709103185. Toxicol Ind Health. 2009. PMID: 19458133 Free PMC article.
-
Coro1B and Coro1C regulate lamellipodia dynamics and cell motility by tuning branched actin turnover.J Cell Biol. 2022 Aug 1;221(8):e202111126. doi: 10.1083/jcb.202111126. Epub 2022 Jun 3. J Cell Biol. 2022. PMID: 35657370 Free PMC article.
-
Change in inflammation in out-patient COPD patients from stable phase to a subsequent exacerbation.Int J Chron Obstruct Pulmon Dis. 2009;4:101-9. doi: 10.2147/copd.s4854. Epub 2009 Apr 15. Int J Chron Obstruct Pulmon Dis. 2009. PMID: 19436694 Free PMC article.
References
-
- Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1:520–4. - PubMed
-
- Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989;140:1745–53. - PubMed
-
- Wilson JW, Bamford TL. Assessing the evidence for remodelling of the airway in asthma. Pulm Pharmacol Ther. 2001;14:229–47. - PubMed
-
- Blyth DI, Wharton TF, Pedrick MS, Savage TJ, Sanjar S. Airway subepithelial fibrosis in a murine model of atopic asthma: suppression by dexamethasone or anti-interleukin-5 antibody. Am J Respir Cell Mol Biol. 2000;23:241–6. - PubMed
-
- Rudd RM, Haslam PL, Turner-Warwick M. Cryptogenic fibrosing alveolitis. Relationships of pulmonary physiology and bronchoalveolar lavage to response to treatment and prognosis. Am Rev Respir Dis. 1981;124:1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

