Elevated expression and release of tissue-type, but not urokinase-type, plasminogen activator after binding of autoantibodies to bullous pemphigoid antigen 180 in cultured human keratinocytes
- PMID: 15008985
- PMCID: PMC1808969
- DOI: 10.1111/j.1365-2249.2004.02401.x
Elevated expression and release of tissue-type, but not urokinase-type, plasminogen activator after binding of autoantibodies to bullous pemphigoid antigen 180 in cultured human keratinocytes
Abstract
In bullous pemphigoid (BP), the binding of BP180-specific antibodies to their hemidesmosomal target antigen is not sufficient for blister formation, but must be accompanied by the release of proteases. Using plasminogen activator (PA) knock-out mice, the PA system has previously been shown to be a prerequisite for blister formation in experimental murine BP. Here, we found elevated levels of plasmin and tPA, but not of uPA, in blister fluid from BP patients (n = 7) compared to blisters from patients with toxic epidermal necrolysis (n = 4) and suction blisters in healthy controls (n = 7). Subsequently, we addressed the question whether keratinocytes release PA in response to the binding of anti-BP180 antibodies. Treatment of cultured normal human keratinocytes with BP IgG, but not with control IgG, led to both increased protein and mRNA levels of tPA, but not of uPA, as determined by ELISA and RT-PCR, respectively. The specificity of this finding was confirmed using BP180-deficient keratinocytes from a patient with generalized atrophic benign epidermolysis bullosa, where no tPA release was observed after stimulation with BP IgG. Our results show the elevated expression and release of tPA from normal human keratinocytes upon stimulation with antibodies to human BP180. Keratinocytes, by secreting tPA, may thus play an active role in blister formation of BP.
Figures

 ), and from blisters in patients with toxic epidermal necrolysis (TEN; n = 4; ▪) were analysed for reactivity with (a) tPA, (b) uPA and (c) plasmin in duplicate by ELISA and chromogenic assay, respectively. Bars show mean ± SD (ng/ml for tPA and uPA, µg/ml for plasmin levels). Asterisks indicate statistical significance between levels in BP blister fluid compared to levels in both blister fluid of SB in healthy volunteers and of blisters in TEN patients (*P < 0·05, **P > 0·01, ***P < 0·001).
), and from blisters in patients with toxic epidermal necrolysis (TEN; n = 4; ▪) were analysed for reactivity with (a) tPA, (b) uPA and (c) plasmin in duplicate by ELISA and chromogenic assay, respectively. Bars show mean ± SD (ng/ml for tPA and uPA, µg/ml for plasmin levels). Asterisks indicate statistical significance between levels in BP blister fluid compared to levels in both blister fluid of SB in healthy volunteers and of blisters in TEN patients (*P < 0·05, **P > 0·01, ***P < 0·001).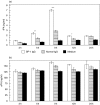
 ), and with medium alone (▪), respectively. After incubation times of 3 h, 6 h, 9 h, 12 h, and 24 h, culture supernatants were analysed for (a) tPA and (b) uPA reactivity in quadruplicate by ELISA. Bars show mean ± SD (ng/ml). Asterisks indicate a statistically significant difference between BP IgG- and normal IgG-treated cells (*P < 0·05, **P > 0·01, ***P < 0·001). This pattern is representative of the pattern seen in 2 separate experiments with keratinocytes from different donors.
), and with medium alone (▪), respectively. After incubation times of 3 h, 6 h, 9 h, 12 h, and 24 h, culture supernatants were analysed for (a) tPA and (b) uPA reactivity in quadruplicate by ELISA. Bars show mean ± SD (ng/ml). Asterisks indicate a statistically significant difference between BP IgG- and normal IgG-treated cells (*P < 0·05, **P > 0·01, ***P < 0·001). This pattern is representative of the pattern seen in 2 separate experiments with keratinocytes from different donors.
 ), and with medium alone (▪) for 9 h. Levels of (a) tPA and (b) uPA in the culture supernatants were analysed in quadruplicate by ELISA. Whereas tPA levels in response to BP IgG were about 3-fold higher compared to treatment with normal human IgG, uPA levels, although about 10-fold higher than tPA levels, were not different between the 2 groups. Bars indicate mean ± SD (ng/ml). Asterisks indicate a statistically significant difference between BP IgG- and normal IgG-treated cells (P < 0·001). This pattern is representative of the pattern seen in 2 separate experiments with keratinocytes from different donors.
), and with medium alone (▪) for 9 h. Levels of (a) tPA and (b) uPA in the culture supernatants were analysed in quadruplicate by ELISA. Whereas tPA levels in response to BP IgG were about 3-fold higher compared to treatment with normal human IgG, uPA levels, although about 10-fold higher than tPA levels, were not different between the 2 groups. Bars indicate mean ± SD (ng/ml). Asterisks indicate a statistically significant difference between BP IgG- and normal IgG-treated cells (P < 0·001). This pattern is representative of the pattern seen in 2 separate experiments with keratinocytes from different donors.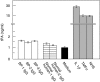
 ), medium alone (▪), and known inducers of tPA, including IL-1β, TNFα, and normal human serum (NHS;
), medium alone (▪), and known inducers of tPA, including IL-1β, TNFα, and normal human serum (NHS;  ). tPA levels were analysed in quadruplicate by ELISA. Bars show mean ± SD (ng/ml).
). tPA levels were analysed in quadruplicate by ELISA. Bars show mean ± SD (ng/ml).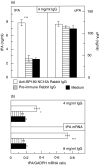
 ), and with medium alone (▪). (a) Culture supernatants were assayed for tPA and uPA reactivity in quadruplicate by ELISA. Bars indicate mean ± SD (ng/ml). (b) tPA mRNA was detected by RT-PCR and expressed as ratio to GAPDH. Asterisks indicate statistical significant difference between R594 IgG- and preimmune IgG-treated cells (*P < 0·05, ***P < 0·001). This pattern is representative of the pattern seen in 2 separate experiments with keratinocytes from different donors.
), and with medium alone (▪). (a) Culture supernatants were assayed for tPA and uPA reactivity in quadruplicate by ELISA. Bars indicate mean ± SD (ng/ml). (b) tPA mRNA was detected by RT-PCR and expressed as ratio to GAPDH. Asterisks indicate statistical significant difference between R594 IgG- and preimmune IgG-treated cells (*P < 0·05, ***P < 0·001). This pattern is representative of the pattern seen in 2 separate experiments with keratinocytes from different donors.Similar articles
-
Autoantibodies to BP180 associated with bullous pemphigoid release interleukin-6 and interleukin-8 from cultured human keratinocytes.J Invest Dermatol. 2000 Nov;115(5):842-8. doi: 10.1046/j.1523-1747.2000.00141.x. J Invest Dermatol. 2000. PMID: 11069622
-
The IL-8 release from cultured human keratinocytes, mediated by antibodies to bullous pemphigoid autoantigen 180, is inhibited by dapsone.Clin Exp Immunol. 2001 Apr;124(1):157-62. doi: 10.1046/j.1365-2249.2001.01503.x. Clin Exp Immunol. 2001. PMID: 11359455 Free PMC article.
-
Synergy between a plasminogen cascade and MMP-9 in autoimmune disease.J Clin Invest. 2005 Apr;115(4):879-87. doi: 10.1172/JCI23977. J Clin Invest. 2005. PMID: 15841177 Free PMC article.
-
Immediate hypersensitivity phenomena in bullous pemphigoid: critical concepts.J Med. 2002;33(1-4):189-98. J Med. 2002. PMID: 12939118 Review.
-
IgE autoantibodies in bullous pemphigoid: supporting role, or leading player?J Dermatol Sci. 2015 Apr;78(1):5-10. doi: 10.1016/j.jdermsci.2015.03.002. Epub 2015 Mar 9. J Dermatol Sci. 2015. PMID: 25797172 Review.
Cited by
-
Comparison of reactivity and epitope recognition between sera from American and Italian patients with oral pemphigoid.Clin Exp Immunol. 2006 Jul;145(1):28-35. doi: 10.1111/j.1365-2249.2006.03103.x. Clin Exp Immunol. 2006. PMID: 16792670 Free PMC article.
-
From Molecular Insights to Clinical Perspectives in Drug-Associated Bullous Pemphigoid.Int J Mol Sci. 2023 Nov 26;24(23):16786. doi: 10.3390/ijms242316786. Int J Mol Sci. 2023. PMID: 38069109 Free PMC article. Review.
-
Proteases in Pemphigoid Diseases.Front Immunol. 2019 Jun 26;10:1454. doi: 10.3389/fimmu.2019.01454. eCollection 2019. Front Immunol. 2019. PMID: 31297118 Free PMC article. Review.
-
14-3-3 sigma isoform interacts with the cytoplasmic domain of the transmembrane BP180 in keratinocytes.J Cell Physiol. 2007 Sep;212(3):675-81. doi: 10.1002/jcp.21064. J Cell Physiol. 2007. PMID: 17443672 Free PMC article.
-
Adaptive and innate immune pathogenesis of bullous pemphigoid: A review.Front Immunol. 2023 Mar 10;14:1144429. doi: 10.3389/fimmu.2023.1144429. eCollection 2023. Front Immunol. 2023. PMID: 36993969 Free PMC article. Review.
References
-
- Stanley JR, Hawley-Nelson P, Yuspa SH, Shevach EM, Katz SI. Characterisation of bullous pemphigoid antigen: a unique basement membrane protein of stratified squamous epithelia. Cell. 1981;24:987–1003. - PubMed
-
- Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol. 1992;99:243–50. - PubMed
-
- Tanaka T, Korman NJ, Shimizu H, Eady RAJ, Klaus-Kovtun V, Cehrs K, Stanley JR. Production of rabbit antibodies against carboxy-terminal epitopes encoded by bullous pemphigoid cDNA. J Invest Dermatol. 1990;94:617–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

