Biologically active neutrophil chemokine pattern in tonsillitis
- PMID: 15008987
- PMCID: PMC1808959
- DOI: 10.1111/j.1365-2249.2003.02390.x
Biologically active neutrophil chemokine pattern in tonsillitis
Abstract
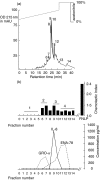
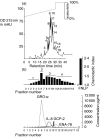
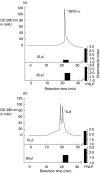
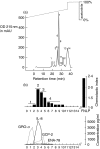
To gain an insight into the mechanisms of chronic and acute inflammation, the production of neutrophil chemokines in different types of tonsillitis - hyperplastic tonsillitis (HT), recurrent tonsillitis (RT) and peritonsillar abscesses (PA) - was investigated. The chemokines interleukin-8 (IL-8), growth-related oncogene-alpha (GRO-alpha), epithelial cell-derived neutrophil attractant-78 (ENA-78) and granulocyte chemotactic protein-2 (GCP-2) were detected and shown to have different biological activities. With respect to the biological properties of CXC chemokines, the biological activity of the chemokines was identified using a three-step high-performance liquid chromatography (HPLC) technique, a bioassay involving measurement of neutrophil chemotaxis in a single Boyden chamber in tissue of HT, RT and PA. Using reverse transcription-polymerase chain reaction (RT-PCR), the chemokine concentrations were determined in the different tonsillitis entities. The chemokine pattern was dominated in PA by IL-8 and GRO-alpha and in RT by GRO-alpha. Hyperplastic tonsils of patients without a history of infection generated about five times lower IL-8 than PA. A protein concentration of GCP-2 was induced in PA and RT, whereas ENA-78 remained the same in all entities. In conclusion, it would appear that IL-8 was up-regulated in acute inflammation, whereas GRO-alpha dominated in chronic inflammation. ENA-78 seems not to play a pivotal role in inflammatory processes in tonsils. GCP-2 may serve as a substitute chemokine in certain inflammatory conditions as its quantity of mRNA and protein was higher in RT and PA than in HT.
Figures


Similar articles
-
Neutrophil chemokines in epithelial inflammatory processes of human tonsils.Clin Exp Immunol. 2005 May;140(2):293-300. doi: 10.1111/j.1365-2249.2005.02773.x. Clin Exp Immunol. 2005. PMID: 15807854 Free PMC article.
-
The primary role in biologic activity of the neutrophil chemokines IL-8 and GRO-alpha in cultured nasal epithelial cells.J Interferon Cytokine Res. 2003 Feb;23(2):113-23. doi: 10.1089/107999003321455507. J Interferon Cytokine Res. 2003. PMID: 12744776
-
Primary role of growth-related oncogene-alpha and granulocyte chemotactic protein-2 as neutrophil chemoattractants in chronic rhinosinusitis.Clin Exp Allergy. 2006 Jun;36(6):748-59. doi: 10.1111/j.1365-2222.2006.02501.x. Clin Exp Allergy. 2006. PMID: 16776676
-
Neutrophil chemokines in cultured nasal fibroblasts.Allergy. 2002 Dec;57(12):1159-64. doi: 10.1034/j.1398-9995.2002.23748.x. Allergy. 2002. PMID: 12464044
-
Granulocyte chemotactic protein-2 and related CXC chemokines: from gene regulation to receptor usage.J Leukoc Biol. 1997 Nov;62(5):563-9. doi: 10.1002/jlb.62.5.563. J Leukoc Biol. 1997. PMID: 9365109 Review.
Cited by
-
IL-8-induced neutrophil chemotaxis is mediated by Janus kinase 3 (JAK3).FEBS Lett. 2011 Jan 3;585(1):159-66. doi: 10.1016/j.febslet.2010.11.031. Epub 2010 Nov 21. FEBS Lett. 2011. PMID: 21095188 Free PMC article.
-
Increased Levels of S100A8/A9 in Patients with Peritonsillar Abscess: A New Promising Diagnostic Marker to Differentiate between Peritonsillar Abscess and Peritonsillitis.Dis Markers. 2017;2017:9126560. doi: 10.1155/2017/9126560. Epub 2017 Oct 17. Dis Markers. 2017. PMID: 29180834 Free PMC article.
-
A chemokine-degrading extracellular protease made by group A Streptococcus alters pathogenesis by enhancing evasion of the innate immune response.Infect Immun. 2008 Mar;76(3):978-85. doi: 10.1128/IAI.01354-07. Epub 2008 Jan 3. Infect Immun. 2008. PMID: 18174342 Free PMC article.
-
Neutrophil chemokines in epithelial inflammatory processes of human tonsils.Clin Exp Immunol. 2005 May;140(2):293-300. doi: 10.1111/j.1365-2249.2005.02773.x. Clin Exp Immunol. 2005. PMID: 15807854 Free PMC article.
-
Combined boyden-flow cytometry assay improves quantification and provides phenotypification of leukocyte chemotaxis.PLoS One. 2011;6(12):e28771. doi: 10.1371/journal.pone.0028771. Epub 2011 Dec 9. PLoS One. 2011. PMID: 22174892 Free PMC article.
References
-
- Luster AD. Mechanisms of disease: chemokines – chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. - PubMed
-
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. 675–705. - PubMed
-
- Luster AD, Cardiff RD, Maclean JA, Crowe K, Granstein RD. Delayed wound healing and disorganized neovascularization in transgenic mice expressing the IP-10 chemokine. Proc Assoc Am Phys. 1998;110:183–96. - PubMed
-
- Mantovani A. Inflammatory cytokines. Recenti Prog Med. 2000;91:422–4. - PubMed
-
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

