Olfactory bulb neurons express functional, entrainable circadian rhythms
- PMID: 15009137
- PMCID: PMC3474850
- DOI: 10.1111/j.0953-816x.2004.03117.x
Olfactory bulb neurons express functional, entrainable circadian rhythms
Abstract
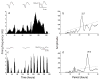
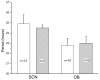
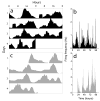
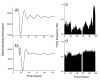
Circadian pacemakers drive many daily molecular, physiological and behavioural rhythms. We investigated whether the main olfactory bulb is a functional circadian pacemaker in rats. Long-term, multielectrode recordings revealed that individual, cultured bulb neurons expressed near 24-h oscillations in firing rate. Real-time recordings of Period1 gene activity showed that a population of cells within the bulb expressed synchronized rhythmicity starting on embryonic day 19. This rhythmicity was intrinsic to the mitral, and not the granule, cell layer, entrainable to physiological temperature cycles and temperature compensated in its period. However, removal of the olfactory bulbs had no effect on running wheel behaviour. These results indicate that individual mitral/tufted cells are competent circadian pacemakers which normally synchronize to each other. The daily rhythms in gene expression and firing rate intrinsic to the olfactory bulb are not required for circadian patterns of locomotion, indicating that they are involved in rhythms outside the canonical circadian system.
Figures

References
-
- Altman PL, Dittmer DS. Biological Handbooks. FASEB; Washington D.C: 1962. Growth, including reproduction and morphological development; pp. 304–313.
-
- Amir S, Cain S, Sullivan J, Robinson B, Stewart J. In rats, odor-induced Fos in the olfactory pathways depends on the phase of the circadian clock. Neurosci Lett. 1999;272:175–178. - PubMed
-
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. - PubMed
-
- Bayer SA. 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res. 1983;50:329–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

