Immunological characterization of tristetraprolin as a low abundance, inducible, stable cytosolic protein
- PMID: 15010466
- PMCID: PMC1351392
- DOI: 10.1074/jbc.M400900200
Immunological characterization of tristetraprolin as a low abundance, inducible, stable cytosolic protein
Abstract
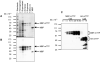
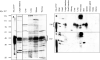
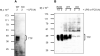
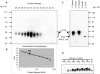
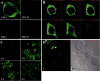
Tristetraprolin (TTP) is a zinc finger protein that can bind to AU-rich elements within certain mRNAs, resulting in deadenylation and destabilization of those mRNAs. Its physiological targets include the mRNAs encoding the cytokines tumor necrosis factor alpha (TNF) and granulocyte-macrophage colony-stimulating factor. TTP was originally identified on the basis of its massive but transient increase in mRNA levels following mitogen stimulation of fibroblasts. It has been difficult to reconcile this transient mRNA profile with the presumed continuing "need" for TTP protein, for example, to reverse the effects of lipopolysaccharide (LPS)-stimulated TNF secretion. To investigate this and other questions concerning endogenous TTP protein in cells and tissues, we raised a high titer rabbit antiserum against full-length mouse TTP. TTP could be detected on immunoblots of mouse cytosolic tissue extracts; it was most highly expressed in spleen, but its concentration in that tissue was only about 1.5 nm. TTP could be detected readily in splenic macrophages and stromal cells from LPS-injected rats. In both LPS-treated RAW 264.7 macrophages and fetal calf serum-treated mouse embryonic fibroblasts, TTP protein was stable after induction, with minimal degradation occurring for several hours after treatment of the cells with cycloheximide. The biosynthesis of TTP was accompanied by large changes in electrophoretic mobility consistent with progressive phosphorylation. Confocal microscopy revealed that TTP accumulated in a vesicular pattern in the cytosol of the LPS-stimulated RAW 264.7 cells, and was occasionally seen in the cytosol of unstimulated dividing cells. Gel filtration of the endogenous protein suggested that its predominant structure was monomeric. TTP appears to be a low abundance, cytosolic protein in unstimulated cells and tissues, but once induced is relatively stable, in contrast to its very labile mRNA.
Figures



References
-
- Blackshear PJ. Biochem. Soc. Trans. 2001;30:945–952. - PubMed
-
- Lai WS, Carballo E, Thorn JM, Kennington EA, Blackshear PJ. J. Biol. Chem. 2000;275:17827–17837. - PubMed
-
- Phillips RS, Ramos SB, Blackshear PJ. J. Biol. Chem. 2002;277:11606–11613. - PubMed
-
- Murata T, Yoshino Y, Morita N, Kaneda N. Biochem. Biophys. Res. Commun. 2002;293:1242–1247. - PubMed
-
- Blackshear PJ, Lai WS, Kennington EA, Brewer G, Wilson GM, Guan X, Zhou P. J. Biol. Chem. 2003;278:19947–19955. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

