Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis
- PMID: 15010700
- PMCID: PMC380968
- DOI: 10.1038/sj.emboj.7600104
Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis
Abstract
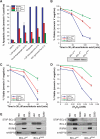
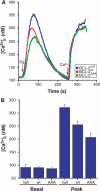
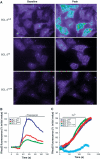
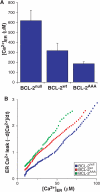
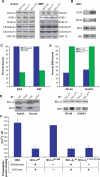
Phosphorylation of BCL-2 within an unstructured loop inhibits its antiapoptotic effect. We found that phosphorylated BCL-2 predominantly localized to the endoplasmic reticulum (ER) and tested whether phosphorylation would control its activity at this organelle, where Ca(2+) dynamics serve as a critical control point for apoptosis. Phosphorylation greatly inhibits the ability of BCL-2 to lower [Ca(2+)](er) and protect against Ca(2+)-dependent death stimuli. Cells expressing nonphosphorylatable BCL-2(AAA) exhibited increased leak of Ca(2+) from the ER and further diminished steady-state [Ca(2+)](er) stores when compared to cells expressing BCL-2(wt). Consequently, when BCL-2 is phosphorylated, Ca(2+) discharge from the ER is increased, with a secondary increase in mitochondrial Ca(2+) uptake. We also demonstrate that phosphorylation of BCL-2 inhibits its binding to proapoptotic family members. This inhibitory mechanism manifested at the ER, where phosphorylated BCL-2 was unable to bind proapoptotic members. [Ca(2+)](er) proved coordinate with the capacity of BCL-2 to bind proapoptotic BH3-only members, further integrating the apoptotic pathway and Ca(2+) modulation. Unexpectedly, the regulation of ER Ca(2+) dynamics is a principal avenue whereby BCL-2 phosphorylation alters susceptibility to apoptosis.
Figures
References
-
- Adams JM, Cory S (2001) Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci 26: 61–66 - PubMed
-
- Bernardi P (1999) Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev 79: 1127–1155 - PubMed
-
- Blagosklonny MV, Giannakakou P, el-Deiry WS, Kingston DG, Higgs PI, Neckers L, Fojo T (1997) Raf-1/bcl-2 phosphorylation: a step from microtubule damage to cell death. Cancer Res 57: 130–135 - PubMed
-
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ (2001) BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell 8: 705–711 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

