Abnormal hippocampal axon bundling in EphB receptor mutant mice
- PMID: 15014111
- PMCID: PMC6729491
- DOI: 10.1523/JNEUROSCI.4711-03.2004
Abnormal hippocampal axon bundling in EphB receptor mutant mice
Abstract
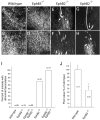
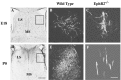
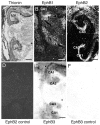
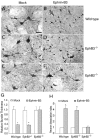
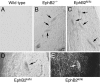
Axons travel frequently in bundles to reach their target. After arriving at the target, axon terminals defasciculate, migrate to topographically defined positions, and form synapses with appropriate target neurons. Here we present evidence that the B-type receptors of the erythropoietin-producing hepatocellular (Eph) family and a ligand, ephrin-B3, influence hippocampal axon defasciculation. The EphB receptors are expressed in the hippocampus, and the ligand, ephrin-B3, is transcribed in the lateral septum, the major subcortical target of hippocampal neurons. Ephrin-B3 promotes adhesion of hippocampal neurons to the ligand-expressing substrates in vitro, and the loss of the receptor EphB2 abrogates the effects of ephrin-B3. In mice deficient in EphB2 and EphB3, many hippocampal axons remain in bundles. This phenotype was also observed in mice that were specifically deleted for the cytoplasmic domain of EphB2. These observations indicate that the EphB receptors and their ligand regulate hippocampal axon defasciculation at the septal target, possibly through a receptor-mediated forward signaling mechanism.
Figures




References
-
- Bergemann AD, Zhang L, Chiang MK, Brambilla R, Klein R, Flanagan JG (1998) Ephrin-B3, a ligand for the receptor EphB3, expressed at the midline of the developing neural tube. Oncogene 16: 471-480. - PubMed
-
- Bohme B, VandenBos T, Cerretti DP, Park LS, Holtrich U, Rubsamen-Waigmann H, Strebhardt K (1996) Cell-cell adhesion mediated by binding of membrane-anchored ligand LERK-2 to the EPH-related receptor human embryonal kinase 2 promotes tyrosine kinase activity. J Biol Chem 271: 24747-24752. - PubMed
-
- Cowan CA, Henkemeyer M (2002) Ephrins in reverse, park and drive. Trends Cell Biol 12: 339-346. - PubMed
-
- Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B (2000) EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron 26: 417-430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
