Alterations in glucose metabolism induce hypothermia leading to tau hyperphosphorylation through differential inhibition of kinase and phosphatase activities: implications for Alzheimer's disease
- PMID: 15014115
- PMCID: PMC6729502
- DOI: 10.1523/JNEUROSCI.5561-03.2004
Alterations in glucose metabolism induce hypothermia leading to tau hyperphosphorylation through differential inhibition of kinase and phosphatase activities: implications for Alzheimer's disease
Abstract
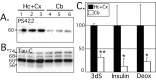
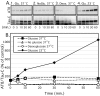
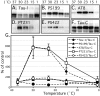
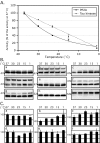
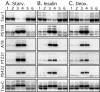
Alzheimer's disease (AD) brains contain neurofibrillary tangles (NFTs) composed of abnormally hyperphosphorylated tau protein. Regional reductions in cerebral glucose metabolism correlating to NFT densities have been reported in AD brains. Assuming that reduced glucose metabolism might cause abnormal tau hyperphosphorylation, we induced in vivo alterations of glucose metabolism in mice by starvation or intraperitoneal injections of either insulin or deoxyglucose. We found that the treatments led to abnormal tau hyperphosphorylation with patterns resembling those in early AD brains and also resulted in hypothermia. Surprisingly, tau hyperphosphorylation could be traced down to a differential effect of low temperatures on kinase and phosphatase activities. These data indicate that abnormal tau hyperphosphorylation is associated with altered glucose metabolism through hypothermia. Our results imply that serine-threonine protein phosphatase 2A plays a major role in regulating tau phosphorylation in the adult brain and provide in vivo evidence for its crucial role in abnormal tau hyperphosphorylation in AD.
Figures



References
-
- Arendt T, Holzer M, Fruth R, Bruckner MK, Gartner U (1998) Phosphorylation of tau, Abeta-formation, and apoptosis after in vivo inhibition of PP-1 and PP-2A. Neurobiol Aging 19: 3-13. - PubMed
-
- Auer RN, Siesjo BK (1993) Hypoglycaemia: brain neurochemistry and neuropathology. Baillieres Clin Endocrinol Metab 7: 611-625. - PubMed
-
- Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM (1989) Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res 477: 90-99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
