Oscillating Purkinje neuron activity causing involuntary eye movement in a mutant mouse deficient in the glutamate receptor delta2 subunit
- PMID: 15014119
- PMCID: PMC6729495
- DOI: 10.1523/JNEUROSCI.0783-03.2004
Oscillating Purkinje neuron activity causing involuntary eye movement in a mutant mouse deficient in the glutamate receptor delta2 subunit
Abstract
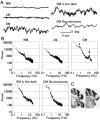
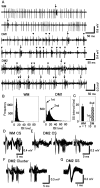
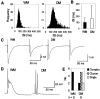
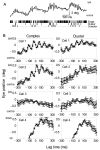
How failures in regulation of synaptic transmission in the mammalian CNS affect neuronal activity and disturb motor coordination is addressed. The mutant mouse deficient in the glutamate receptor delta2 subunit, specifically expressed in cerebellar Purkinje neurons, has defects in synaptic regulations such as synaptic plasticity, stabilization, and elimination of synaptic connections and shows failures in motor coordination and learning. In this study, the cause of motor discoordination of the delta2 mutant mouse was analyzed by comparing its motor control ability with those of the wild-type mouse and the lurcher mutant mouse, which loses all Purkinje neurons, the sole output neurons in the cerebellar cortex. Unexpectedly, the delta2 mutant mouse showed severer motor discoordination than the lurcher mouse without any cerebellar cortical outputs. The delta2 mutant mouse showed involuntary spontaneous eye movement with characteristic 10 Hz oscillation, which disappeared by ablation of the cerebellar flocculus, suggesting that the delta2 mutant cerebellar cortex outputs an abnormal signal. In vivo extracellular recordings of neuronal activity revealed that Purkinje neurons tended to fire clustered action potentials and complex spikes at approximately 10 Hz in the delta2 mutant mouse. A whole-cell patch-clamp recording from Purkinje neurons in cerebellar slices indicated that the clustered action potentials could be induced by climbing fiber activation. Taken together, our results suggest that the delta2 subunit deficiency produces the oscillating activity in Purkinje neurons by enhancing climbing fiber inputs, causing surplus movement and affecting motor control worse than no signal at all.
Figures





References
-
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S (1994) Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell 7: 377-388. - PubMed
-
- Aizenman CD, Linden DJ (2000) Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci 3: 109-111. - PubMed
-
- Araki K, Meguro H, Kushiya E, Takayama C, Inoue Y, Mishina M (1993) Selective expression of the glutamate receptor channel δ2 subunit in cerebellar Purkinje cells. Biochem Biophys Res Commun 197: 1267-1276. - PubMed
-
- Chen C, Kano M, Abeliovich A, Chen L, Bao S, Kim JJ, Hashimoto K, Thompson RF, Tonegawa S (1995) Impaired motor coordination correlates with persistent multiple climbing fiber innervation in PKCγ mutant mice. Cell 83: 1233-1242. - PubMed
-
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Conde F, Collingridge GL, Crepel F (1994) Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature 372: 237-243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
