The two regulatory subunits of aplysia cAMP-dependent protein kinase mediate distinct functions in producing synaptic plasticity
- PMID: 15014122
- PMCID: PMC6729487
- DOI: 10.1523/JNEUROSCI.4331-03.2004
The two regulatory subunits of aplysia cAMP-dependent protein kinase mediate distinct functions in producing synaptic plasticity
Abstract
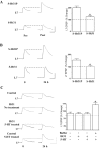
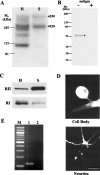
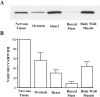
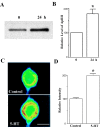
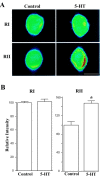
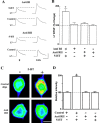
Activation of the cAMP-dependent protein kinase (PKA) is critical for both short- and long-term facilitation in Aplysia sensory neurons. There are two types of the kinase, I and II, differing in their regulatory (R) subunits. We cloned Aplysia RII; RI was cloned previously. Type I PKA is mostly soluble in the cell body whereas type II is enriched at nerve endings where it is bound to two prominent A kinase-anchoring-proteins (AKAPs). Disruption of the binding of RII to AKAPs by Ht31, an inhibitory peptide derived from a human thyroid AKAP, prevents both the short- and the long-term facilitation produced by serotonin (5-HT). During long-term facilitation, RII is transcriptionally upregulated; in contrast, the amount of RI subunits decreases, and previous studies have indicated that the decrease is through ubiquitin-proteosome-mediated proteolysis. Experiments with antisense oligonucleotides injected into the sensory neuron cell body show that the increase in RII protein is essential for the production of long-term facilitation. Using synaptosomes, we found that 5-HT treatment causes RII protein to increase at nerve endings. In addition, using reverse transcription-PCR, we found that RII mRNA is transported from the cell body to nerve terminals. Our results suggest that type I operates in the nucleus to maintain cAMP response element-binding protein-dependent gene expression, and type II PKA acts at sensory neuron synapses phosphorylating proteins to enhance release of neurotransmitter. Thus, the two types of the kinase have distinct but complementary functions in the production of facilitation at synapses of an identified neuron.
Figures

Similar articles
-
Serotonin induces selective cleavage of the PKA RI subunit but not RII subunit in Aplysia neurons.Biochem Biophys Res Commun. 2007 Aug 3;359(3):563-7. doi: 10.1016/j.bbrc.2007.05.146. Epub 2007 May 30. Biochem Biophys Res Commun. 2007. PMID: 17548057 Free PMC article.
-
Alpha/beta-tubulin are A kinase anchor proteins for type I PKA in neurons.Brain Res. 2009 Jan 28;1251:53-64. doi: 10.1016/j.brainres.2008.11.019. Epub 2008 Nov 18. Brain Res. 2009. PMID: 19056362
-
Serotonin regulates the secretion and autocrine action of a neuropeptide to activate MAPK required for long-term facilitation in Aplysia.Neuron. 2004 Aug 5;43(3):373-85. doi: 10.1016/j.neuron.2004.07.011. Neuron. 2004. PMID: 15294145
-
Cellular, molecular, and epigenetic mechanisms in non-associative conditioning: implications for pain and memory.Neurobiol Learn Mem. 2013 Oct;105:133-50. doi: 10.1016/j.nlm.2013.06.008. Epub 2013 Jun 22. Neurobiol Learn Mem. 2013. PMID: 23796633 Free PMC article. Review.
-
Presynaptic facilitation revisited: state and time dependence.J Neurosci. 1996 Jan 15;16(2):425-35. doi: 10.1523/JNEUROSCI.16-02-00425.1996. J Neurosci. 1996. PMID: 8551327 Free PMC article. Review.
Cited by
-
Inhibition of neurotransmitter release by a nonphysiological target requires protein synthesis and involves cAMP-dependent and mitogen-activated protein kinases.J Neurosci. 2004 May 26;24(21):5054-62. doi: 10.1523/JNEUROSCI.5671-03.2004. J Neurosci. 2004. PMID: 15163698 Free PMC article.
-
Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation.Exp Neurol. 2009 May;217(1):116-23. doi: 10.1016/j.expneurol.2009.01.017. Epub 2009 Feb 3. Exp Neurol. 2009. PMID: 19416672 Free PMC article.
-
Role for A kinase-anchoring proteins (AKAPS) in glutamate receptor trafficking and long term synaptic depression.J Biol Chem. 2005 Apr 29;280(17):16962-16968. doi: 10.1074/jbc.M409693200. Epub 2005 Feb 17. J Biol Chem. 2005. PMID: 15718245 Free PMC article.
-
Protein kinase C regulates local synthesis and secretion of a neuropeptide required for activity-dependent long-term synaptic plasticity.J Neurosci. 2007 Aug 15;27(33):8927-39. doi: 10.1523/JNEUROSCI.2322-07.2007. J Neurosci. 2007. PMID: 17699674 Free PMC article.
-
The AKAP Yu is required for olfactory long-term memory formation in Drosophila.Proc Natl Acad Sci U S A. 2007 Aug 21;104(34):13792-7. doi: 10.1073/pnas.0700439104. Epub 2007 Aug 9. Proc Natl Acad Sci U S A. 2007. PMID: 17690248 Free PMC article.
References
-
- Alberini CM, Ghirardi M, Metz R, Kandel ER (1994) C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell 76: 1099-1114. - PubMed
-
- Amieux PS, McKnight GS (2002) The essential role of RI alpha in the maintenance of regulated PKA activity. Ann NY Acad Sci 968: 75-95. - PubMed
-
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER (1995) Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell 83: 979-992. - PubMed
-
- Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER (1998) CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell 95: 211-223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
