Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice
- PMID: 15014128
- PMCID: PMC6729489
- DOI: 10.1523/JNEUROSCI.4315-03.2004
Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice
Abstract
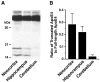
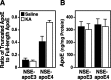
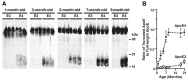
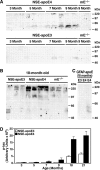
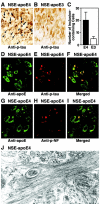
Apolipoprotein E (apoE) is found in amyloid plaques and neurofibrillary tangles (NFTs) in Alzheimer's disease (AD) brains, but its role in their pathogenesis is unclear. Previously, we found C-terminal-truncated fragments of apoE in AD brains and showed that such fragments can cause neurodegeneration and can induce NFT-like inclusions in cultured neuronal cells and in transgenic mice. Here, we analyzed apoE fragmentation in brain tissue homogenates from transgenic mice expressing apoE3 or apoE4 in neurons [neuron-specific enolase (NSE)-apoE] or astrocytes [glial fibrillary acidic protein (GFAP)-apoE] by Western blotting. The C-terminal-truncated fragments of apoE accumulated, in an age-dependent manner, in the brains of NSE-apoE4 and, to a significantly lesser extent, NSE-apoE3 mice; however, no fragments were detected in GFAP-apoE3 or GFAP-apoE4 mice. In NSE-apoE mice, the pattern of apoE fragmentation resembled that seen in AD brains, and the fragmentation was specific for certain brain regions, occurring in the neocortex and hippocampus, which are vulnerable to AD-related neurodegeneration, but not in the less vulnerable cerebellum. Excitotoxic challenge with kainic acid significantly increased apoE fragmentation in NSE-apoE4 but not NSE-apoE3 mice. Phosphorylated tau (p-tau) also accumulated in an age-dependent manner in NSE-apoE4 mice and, to a much lesser extent, in NSE-apoE3 mice but not in GFAP-apoE3 or GFAP-apoE4 mice. Intraneuronal p-tau inclusions in the hippocampus were prominent in 21-month-old NSE-apoE4 mice but barely detectable in NSE-apoE3 mice. Thus, the accumulation of potentially pathogenic C-terminal-truncated fragments of apoE depends on both the isoform and the cellular source of apoE. Neuron-specific proteolytic cleavage of apoE4 is associated with increased phosphorylation of tau and may play a key role in the development of AD-related neuronal deficits.
Figures

References
-
- Aoki K, Uchihara T, Sanjo N, Nakamura A, Ikeda K, Tsuchiya K, Wakayama Y (2003) Increased expression of neuronal apolipoprotein E in human brain with cerebral infarction. Stroke 34: 875-880. - PubMed
-
- Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, Ghetti B, Paul SM (1999) Apolipoprotein E is essential for amyloid deposition in the APPV717F transgenic mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 96: 15233-15238. - PMC - PubMed
-
- Bao F, Arai H, Matsushita S, Higuchi S, Sasaki H (1996) Expression of apolipoprotein E in normal and diverse neurodegenerative disease brain. NeuroReport 7: 1733-1739. - PubMed
-
- Beffert U, Poirier J (1996) Apolipoprotein E, plaques, tangles and cholinergic dysfunction in Alzheimer's disease. Ann NY Acad Sci 777: 166-174. - PubMed
-
- Boschert U, Merlo-Pich E, Higgins G, Roses AD, Catsicas S (1999) Apolipoprotein E expression by neurons surviving excitotoxic stress. Neurobiol Dis 6: 508-514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
