Styrylbenzoxazole derivatives for in vivo imaging of amyloid plaques in the brain
- PMID: 15014129
- PMCID: PMC6729476
- DOI: 10.1523/JNEUROSCI.4456-03.2004
Styrylbenzoxazole derivatives for in vivo imaging of amyloid plaques in the brain
Abstract
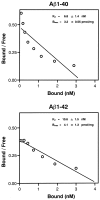
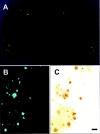
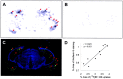
Progressive deposition of senile plaques (SPs) is one of the major neuropathological features of Alzheimer's disease (AD) that precedes cognitive decline. Noninvasive detection of SPs could, therefore, be a potential diagnostic test for early detection of AD patients. For imaging SPs in the living brain, we have developed a series of styrylbenzoxazole derivatives that achieve high binding affinity for amyloid-beta (Abeta) fibrils. One of these compounds, 6-(2-Fluoroethoxy)-2-[2-(4-methylaminophenil) ethenyl]benzoxazole (BF-168), selectively binds SPs in AD brain sections and recognizes Abeta1-42-positive diffuse plaques as well as neuritic plaques in AD brain sections. Intravenous injection of BF-168 in PS1/APP and APP23 transgenic mice resulted in specific in vivo labeling to both compact and diffuse amyloid deposits in the brain. In addition, (18)F-radiolabeled BF-168 demonstrated abundant initial brain uptake (3.9% injected dose/gm at 2 min after injection) and fast clearance (t(1/2) = 24.7 min) after intravenous administration in normal mice. Furthermore, autoradiograms of brain sections from APP23 transgenic mice at 180 min after intravenous injection of [(18)F]BF-168 showed selective labeling of brain amyloid deposits with little nonspecific binding. These findings strongly suggest that styrylbenzoxazole derivatives are promising candidate probes for positron emission tomography and single-photon emission computed tomography imaging for early detection of amyloid plaque formation.
Figures




Similar articles
-
A novel imaging probe for in vivo detection of neuritic and diffuse amyloid plaques in the brain.J Mol Neurosci. 2004;24(2):247-55. doi: 10.1385/JMN:24:2:247. J Mol Neurosci. 2004. PMID: 15456938
-
Detection of amyloid plaques by radioligands for Abeta40 and Abeta42: potential imaging agents in Alzheimer's patients.J Mol Neurosci. 2003 Feb;20(1):15-24. doi: 10.1385/JMN:20:1:15. J Mol Neurosci. 2003. PMID: 12663930
-
Development of amyloid imaging PET probes for an early diagnosis of Alzheimer's disease.Minim Invasive Ther Allied Technol. 2006;15(4):209-13. doi: 10.1080/13645700600836000. Minim Invasive Ther Allied Technol. 2006. PMID: 16966133
-
[Development of SPECT Probes for In Vivo Imaging of β-Amyloid and Tau Aggregates in the Alzheimer's Disease Brain].Yakugaku Zasshi. 2017;137(11):1361-1365. doi: 10.1248/yakushi.17-00156. Yakugaku Zasshi. 2017. PMID: 29093372 Review. Japanese.
-
6-(2-[18F]Fluoroethoxy)-2-[2-(4-methylaminophenyl)ethenyl]benzoxazole.2006 Oct 3 [updated 2008 Jan 28]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2006 Oct 3 [updated 2008 Jan 28]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641407 Free Books & Documents. Review.
Cited by
-
Amyloid-β probes: Review of structure-activity and brain-kinetics relationships.Beilstein J Org Chem. 2013 May 28;9:1012-44. doi: 10.3762/bjoc.9.116. Print 2013. Beilstein J Org Chem. 2013. PMID: 23766818 Free PMC article.
-
Ligands for Protein Fibrils of Amyloid-β, α-Synuclein, and Tau.Chem Rev. 2025 Jun 11;125(11):5282-5348. doi: 10.1021/acs.chemrev.4c00838. Epub 2025 May 6. Chem Rev. 2025. PMID: 40327808 Free PMC article. Review.
-
Selective interaction of lansoprazole and astemizole with tau polymers: potential new clinical use in diagnosis of Alzheimer's disease.J Alzheimers Dis. 2010;19(2):573-89. doi: 10.3233/JAD-2010-1262. J Alzheimers Dis. 2010. PMID: 20110603 Free PMC article.
-
Synthesis, Characterization and Metal Ion Detection of Novel Fluoroionophores Based on Heterocyclic Substituted Alanines.Sensors (Basel). 2007 Oct 3;7(10):2096-2114. doi: 10.3390/s7102096. Sensors (Basel). 2007. PMID: 28903216 Free PMC article.
-
A 18F-labeled BF-227 derivative as a potential radioligand for imaging dense amyloid plaques by positron emission tomography.Mol Imaging Biol. 2013 Aug;15(4):497-506. doi: 10.1007/s11307-012-0608-5. Mol Imaging Biol. 2013. PMID: 23362000
References
-
- Agdeppa ED, Kepe V, Liu J, Flores-Torres S, Satyamurthy N, Petric A, Cole GM, Small GW, Huang SC, Barrio JR (2001) Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for beta-amyloid plaques in Alzheimer's disease. J Neurosci 21: RC189(1-5). - PMC - PubMed
-
- American Psychiatric Association (1994) Diagnostic and statistical manuals of mental disorders, Ed 4. Washington, DC: American Psychiatric Association.
-
- Bacskai BJ, Klunk WE, Mathis CA, Hyman BT (2002) Imaging amyloid-beta deposits in vivo. J Cereb Blood Flow Metab 22: 1035-1041. - PubMed
-
- Cheng Y, Prusoff WH (1973) Relationship between the inhibition constant (Ki) and the concentration of an inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22: 3099-3108. - PubMed
-
- Esiri MM (2001) The neuropathology of Alzheimer's disease. In: Neurobiology of Alzheimer's disease, Ed 2 (Dawbarn D, Allen SJ, eds), pp 33-53. New York: Oxford UP.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
