Loss-of-function analysis of EphA receptors in retinotectal mapping
- PMID: 15014130
- PMCID: PMC6729493
- DOI: 10.1523/JNEUROSCI.0239-03.2004
Loss-of-function analysis of EphA receptors in retinotectal mapping
Abstract
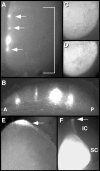
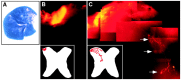
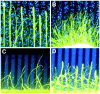
EphA tyrosine kinases are thought to act as topographically specific receptors in the well-characterized projection map from the retina to the tectum. Here, we describe a loss-of-function analysis of EphA receptors in retinotectal mapping. Expressing patches of a cytoplasmically truncated EphA3 receptor in chick retina caused temporal axons to have reduced responsiveness to posterior tectal repellent activity in vitro and to shift more posteriorly within the map in vivo. A gene disruption of mouse EphA5, replacing the intracellular domain with beta-galactosidase, reduced in vitro responsiveness of temporal axons to posterior target membranes. It also caused map abnormalities in vivo, with temporal axons shifted posteriorly and nasal axons anteriorly, but with the entire target still filled by retinal axons. The anterior shift of nasal axons was not accompanied by increased responsiveness to tectal repellent activity, in contrast to the comparable anterior shift in ephrin-A knock-outs, helping to resolve a previous ambiguity in interpreting the ephrin gene knock-outs. The results show the functional requirement for endogenous EphA receptors in retinotectal mapping, show that the receptor intracellular domain is required for a forward signaling response to topographic cues, and provide new evidence for a role of axon competition in topographic mapping.
Figures



References
-
- Birgbauer E, Cowan CA, Sretavan DW, Henkemeyer M (2000) Kinase independent function of EphB receptors in retinal axon pathfinding to the optic disc from dorsal but not ventral retina. Development 127: 1231-1241. - PubMed
-
- Brown A, Yates PA, Burrola P, Ortuno D, Vaidya A, Jessell TM, Pfaff SL, O'Leary DDM, Lemke G (2000) Topographic mapping from the retina to the midbrain is controlled by relative but not absolute levels of EphA receptor signaling. Cell 102: 77-88. - PubMed
-
- Bruckner K, Pasquale EB, Klein R (1997) Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science 275: 1640-1643. - PubMed
-
- Cheng H-J, Nakamoto M, Bergemann AD, Flanagan JG (1995) Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell 82: 371-381. - PubMed
-
- Ciossek T, Lerch MM, Ulrich A (1995) Cloning, characterization, and differential expresssion of MDK2 and MDK5, two novel receptor tyrosine kinases of the eck/eph family. Oncogene 11: 2085-2095. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
