Zaleplon, A Novel Nonbenzodiazepine Hypnotic, Effectively Treats Insomnia in Elderly Patients Without Causing Rebound Effects
- PMID: 15014684
- PMCID: PMC181075
- DOI: 10.4088/pcc.v01n0404
Zaleplon, A Novel Nonbenzodiazepine Hypnotic, Effectively Treats Insomnia in Elderly Patients Without Causing Rebound Effects
Abstract
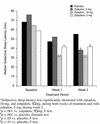
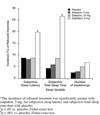
BACKGROUND: Insomnia is a very common symptom, particularly in the elderly. Thus, all hypnotic medications should be carefully evaluated in the elderly population. Zaleplon, a new nonbenzodiazepine hypnotic with a short elimination half-life (approximately 1 hour), was evaluated in the current study. METHOD: This multicenter, randomized, placebo-controlled outpatient study evaluated the efficacy and safety of zaleplon, 5 and 10 mg, in elderly patients with insomnia (as defined by DSM-IV); zolpidem, 5 mg, was the active comparator. Sleep was assessed in 549 elderly patients (>/= 65 years old) by using morning questionnaires completed after each of 7 baseline nights during which placebo was given, 14 nights of double-blind treatment, and 7 nights of placebo after discontinuation of active treatment. RESULTS: Zaleplon, 10 mg, and zolpidem, 5 mg, significantly reduced sleep latency during both weeks of the study. Zaleplon, 5 mg, reduced sleep latency only during week 2. Sleep duration was increased with zolpidem, 5 mg, during weeks 1 and 2 and with zaleplon, 10 mg, during week 1. No clinically significant rebound insomnia was observed after discontinuation of treatment with zaleplon, whereas evidence of rebound effects was seen with zolpidem. There was no significant difference between either zaleplon dose and placebo in the frequency of any central nervous system adverse events. CONCLUSION: Zaleplon is effective in reducing latency to sleep without evidence of undesired effects in elderly patients with insomnia.
Figures

References
-
- Dement WC, Miles LE, Carskadon MA. “White paper” on sleep and aging. J Am Geriatr Soc. 1982;30:25–50. - PubMed
-
- Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–432. - PubMed
-
- Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment: prevalence and correlates. Arch Gen Psychiatry. 1985;42:225–232. - PubMed
-
- Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation survey I. Sleep. 1999;22(suppl 2):S347–S353. - PubMed
-
- Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation survey I. Sleep. 1999;22(suppl 2):S354–S358. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
