Escitalopram: A New SSRI for the Treatment of Depression in Primary Care
- PMID: 15014711
- PMCID: PMC315490
- DOI: 10.4088/pcc.v04n0601
Escitalopram: A New SSRI for the Treatment of Depression in Primary Care
Abstract
Escitalopram is the S-enantiomer of the racemic compound citalopram, a selective serotonin reuptake inhibitor (SSRI) widely used for the treatment of depression. This review describes the current body of pharmacologic and clinical evidence supporting the use of escitalopram for the treatment of depression and anxiety. Preclinical studies have confirmed that it is primarily this molecule that provides the inhibition of serotonin reuptake responsible for the antidepressant effect of citalopram, with minimal-to-nonexistent affinity for other receptor sites. Clinical trials of escitalopram in depressed patients indicate that escitalopram, 10 mg/day, is as effective as 40 mg/day of its parent compound, citalopram, with an excellent safety and tolerability profile. Because of its increased selectivity, escitalopram represents a refinement in SSRI therapy for symptoms of depression and anxiety. This article also explores the implications of a more selective SSRI on the management of depressed patients in the primary care clinical practice.
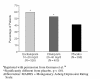
Figures
Similar articles
-
Escitalopram: a second-generation SSRI.CNS Spectr. 2002 Apr;7(4 Suppl 1):34-9. doi: 10.1017/s1092852900028583. CNS Spectr. 2002. PMID: 15131491
-
Escitalopram for the treatment of major depression and anxiety disorders.Expert Rev Neurother. 2008 Apr;8(4):537-52. doi: 10.1586/14737175.8.4.537. Expert Rev Neurother. 2008. PMID: 18416657 Review.
-
Escitalopram: a unique mechanism of action.Int J Psychiatry Clin Pract. 2004;8 Suppl 1:11-3. doi: 10.1080/13651500410005496. Int J Psychiatry Clin Pract. 2004. PMID: 24930683
-
Escitalopram: a pharmacoeconomic review of its use in depression.Pharmacoeconomics. 2003;21(16):1185-209. doi: 10.2165/00019053-200321160-00004. Pharmacoeconomics. 2003. PMID: 14594439 Review.
-
Efficacy comparison of escitalopram and citalopram in the treatment of major depressive disorder: pooled analysis of placebo-controlled trials.CNS Spectr. 2002 Apr;7(4 Suppl 1):40-4. doi: 10.1017/s1092852900028595. CNS Spectr. 2002. PMID: 15131492
Cited by
-
Lower sertraline plasma concentration in patients co-medicated with clozapine-Implications for pharmacological augmentation strategies in schizophrenia.Pharmacol Res Perspect. 2023 Apr;11(2):e01065. doi: 10.1002/prp2.1065. Pharmacol Res Perspect. 2023. PMID: 36825450 Free PMC article.
-
The Effect of Citalopram on Genome-Wide DNA Methylation of Human Cells.Int J Genomics. 2018 Jul 25;2018:8929057. doi: 10.1155/2018/8929057. eCollection 2018. Int J Genomics. 2018. PMID: 30148158 Free PMC article.
-
Predicting Treatment Outcome in Major Depressive Disorder Using Serotonin 4 Receptor PET Brain Imaging, Functional MRI, Cognitive-, EEG-Based, and Peripheral Biomarkers: A NeuroPharm Open Label Clinical Trial Protocol.Front Psychiatry. 2020 Jul 23;11:641. doi: 10.3389/fpsyt.2020.00641. eCollection 2020. Front Psychiatry. 2020. PMID: 32792991 Free PMC article.
-
The role of cytochromes P450 in the metabolism of selected antidepressants and anxiolytics under psychological stress.Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2022 May;166(2):140-149. doi: 10.5507/bp.2022.019. Epub 2022 Apr 12. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2022. PMID: 35438085 Review.
-
Decreased Brain Ventricular Volume in Psychiatric Inpatients with Serotonin Reuptake Inhibitor Treatment.Chronic Stress (Thousand Oaks). 2022 Jul 14;6:24705470221111092. doi: 10.1177/24705470221111092. eCollection 2022 Jan-Dec. Chronic Stress (Thousand Oaks). 2022. PMID: 35859799 Free PMC article.
References
-
- Owens MJ, Rosenbaum JF. Escitalopram: a second-generation SSRI. CNS Spectrums. 2002;7(suppl 1):34–39. - PubMed
-
- Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50:345–350. - PubMed
-
- Hytell J, Bøgesø KP, Perregaard J, et. al. The pharmacological effect of citalopram resides in the (S)-(+)-enantiomer. J Neural Transm. 1992;88:157–160. - PubMed
-
- Sanchez C, Hogg S. The antidepressant effect of citalopram resides in the S-enantiomer (Lu 26-054) Biol Psychiatry. 2000;47:88S.
-
- Mork A, Kreilgaard M, Sanchez C, and et. al. Escitalopram: a comparative study of in vitro and in vivo 5-HT uptake inhibitory activity [poster]. Presented at the 23rd Congress of the Collegium Internationale Neuro-Psychopharmacologium; June 23–27, 2002; Montreal, Canada. P.1.E.054.
LinkOut - more resources
Full Text Sources
Medical
