Thimerosal stimulates Ca2+ flux through inositol 1,4,5-trisphosphate receptor type 1, but not type 3, via modulation of an isoform-specific Ca2+-dependent intramolecular interaction
- PMID: 15015936
- PMCID: PMC1133765
- DOI: 10.1042/BJ20040072
Thimerosal stimulates Ca2+ flux through inositol 1,4,5-trisphosphate receptor type 1, but not type 3, via modulation of an isoform-specific Ca2+-dependent intramolecular interaction
Abstract
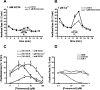
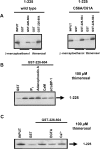
Thiol-reactive agents such as thimerosal have been shown to modulate the Ca2+-flux properties of IP3 (inositol 1,4,5-trisphosphate) receptor (IP3R) via an as yet unidentified mechanism [Parys, Missiaen, De Smedt, Droogmans and Casteels (1993) Pflügers Arch. 424, 516-522; Kaplin, Ferris, Voglmaier and Snyder (1994) J. Biol. Chem. 269, 28972-28978; Missiaen, Taylor and Berridge (1992) J. Physiol. (Cambridge, U.K.) 455, 623-640; Missiaen, Parys, Sienaert, Maes, Kunzelmann, Takahashi, Tanzawa and De Smedt (1998) J. Biol. Chem. 273, 8983-8986]. In the present study, we show that thimerosal potentiated IICR (IP3-induced Ca2+ release) and IP3-binding activity of IP3R1, expressed in triple IP3R-knockout R23-11 cells derived from DT40 chicken B lymphoma cells, but not of IP3R3 or [D1-225]-IP3R1, which lacks the N-terminal suppressor domain. Using a 45Ca2+-flux technique in permeabilized A7r5 smooth-muscle cells, we have shown that Ca2+ shifted the stimulatory effect of thimerosal on IICR to lower concentrations of thimerosal and thereby increased the extent of Ca2+ release. This suggests that Ca2+ and thimerosal synergetically regulate IP3R1. Glutathione S-transferase pull-down experiments elucidated an interaction between amino acids 1-225 (suppressor domain) and amino acids 226-604 (IP3-binding core) of IP3R1, and this interaction was strengthened by both Ca2+ and thimerosal. In contrast, calmodulin and sCaBP-1 (short Ca2+-binding protein-1), both having binding sites in the 1-225 region, weakened the interaction. This interaction was not found for IP3R3, in agreement with the lack of functional stimulation of this isoform by thimerosal. The interaction between the IP3-binding and transmembrane domains (amino acids 1-604 and 2170-2749 respectively) was not affected by thimerosal and Ca2+, but it was significantly inhibited by IP3 and adenophostin A. Our results demonstrate that thimerosal and Ca2+ induce isoform-specific conformational changes in the N-terminal part of IP3R1, leading to the formation of a highly IP3-sensitive Ca2+-release channel.
Figures




Similar articles
-
Ca2+ and calmodulin differentially modulate myo-inositol 1,4, 5-trisphosphate (IP3)-binding to the recombinant ligand-binding domains of the various IP3 receptor isoforms.Biochem J. 2000 Mar 1;346 Pt 2(Pt 2):275-80. Biochem J. 2000. PMID: 10677344 Free PMC article.
-
A calmodulin antagonist reveals a calmodulin-independent interdomain interaction essential for activation of inositol 1,4,5-trisphosphate receptors.Biochem J. 2008 Dec 1;416(2):243-53. doi: 10.1042/BJ20080861. Biochem J. 2008. PMID: 18637794
-
Modulation of inositol 1,4,5-trisphosphate binding to the various inositol 1,4,5-trisphosphate receptor isoforms by thimerosal and cyclic ADP-ribose.Biochem Pharmacol. 2001 Apr 1;61(7):803-9. doi: 10.1016/s0006-2952(01)00540-8. Biochem Pharmacol. 2001. PMID: 11274965
-
[IP3 receptor, a Ca2+ oscilator--role of IP3 receptor in development and neural plasticity].Nihon Yakurigaku Zasshi. 2002 Nov;120(1):6P-10P. Nihon Yakurigaku Zasshi. 2002. PMID: 12491767 Review. Japanese.
-
Inositol 1,4,5-trisphosphate receptors as signal integrators.Annu Rev Biochem. 2004;73:437-65. doi: 10.1146/annurev.biochem.73.071403.161303. Annu Rev Biochem. 2004. PMID: 15189149 Review.
Cited by
-
Redox regulation of type-I inositol trisphosphate receptors in intact mammalian cells.J Biol Chem. 2018 Nov 9;293(45):17464-17476. doi: 10.1074/jbc.RA118.005624. Epub 2018 Sep 18. J Biol Chem. 2018. PMID: 30228182 Free PMC article.
-
Regulation of inositol 1,4,5-trisphosphate receptor type 1 function during oocyte maturation by MPM-2 phosphorylation.Cell Calcium. 2009 Jul;46(1):56-64. doi: 10.1016/j.ceca.2009.04.004. Epub 2009 May 30. Cell Calcium. 2009. PMID: 19482353 Free PMC article.
-
Uncoupling of ATP-mediated calcium signaling and dysregulated interleukin-6 secretion in dendritic cells by nanomolar thimerosal.Environ Health Perspect. 2006 Jul;114(7):1083-91. doi: 10.1289/ehp.8881. Environ Health Perspect. 2006. PMID: 16835063 Free PMC article.
-
Bcl-xL acts as an inhibitor of IP3R channels, thereby antagonizing Ca2+-driven apoptosis.Cell Death Differ. 2022 Apr;29(4):788-805. doi: 10.1038/s41418-021-00894-w. Epub 2021 Nov 8. Cell Death Differ. 2022. PMID: 34750538 Free PMC article.
-
Ins(1,4,5)P3 receptor-mediated Ca2+ signaling and autophagy induction are interrelated.Autophagy. 2011 Dec;7(12):1472-89. doi: 10.4161/auto.7.12.17909. Autophagy. 2011. PMID: 22082873 Free PMC article.
References
-
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature (London) 1993;361:315–325. - PubMed
-
- Furuichi T., Mikoshiba K. Inositol 1,4,5-trisphosphate receptor-mediated Ca2+ signaling in the brain. J. Neurochem. 1995;64:953–960. - PubMed
-
- Yoshikawa F., Iwasaki H., Michikawa T., Furuichi T., Mikoshiba K. Trypsinized cerebellar inositol 1,4,5-trisphosphate receptor. Structural and functional coupling of cleaved ligand binding and channel domains. J. Biol. Chem. 1999;274:316–327. - PubMed
-
- Uchida K., Miyauchi H., Furuichi T., Michikawa T., Mikoshiba K. Critical regions for activation gating of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 2003;278:16551–16560. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

