The Ras/cAMP-dependent protein kinase signaling pathway regulates an early step of the autophagy process in Saccharomyces cerevisiae
- PMID: 15016820
- PMCID: PMC1705971
- DOI: 10.1074/jbc.M400272200
The Ras/cAMP-dependent protein kinase signaling pathway regulates an early step of the autophagy process in Saccharomyces cerevisiae
Abstract
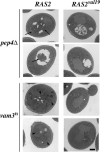
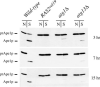
When faced with nutrient deprivation, Saccharomyces cerevisiae cells enter into a nondividing resting state, known as stationary phase. The Ras/PKA (cAMP-dependent protein kinase) signaling pathway plays an important role in regulating the entry into this resting state and the subsequent survival of stationary phase cells. The survival of these resting cells is also dependent upon autophagy, a membrane trafficking pathway that is induced upon nutrient deprivation. Autophagy is responsible for targeting bulk protein and other cytoplasmic constituents to the vacuolar compartment for their ultimate degradation. The data presented here demonstrate that the Ras/PKA signaling pathway inhibits an early step in autophagy because mutants with elevated levels of Ras/PKA activity fail to accumulate transport intermediates normally associated with this process. Quantitative assays indicate that these increased levels of Ras/PKA signaling activity result in an essentially complete block to autophagy. Interestingly, Ras/PKA activity also inhibited a related process, the cytoplasm to vacuole targeting (Cvt) pathway that is responsible for the delivery of a subset of vacuolar proteins in growing cells. These data therefore indicate that the Ras/PKA signaling pathway is not regulating a switch between the autophagy and Cvt modes of transport. Instead, it is more likely that this signaling pathway is controlling an activity that is required during the early stages of both of these membrane trafficking pathways. Finally, the data suggest that at least a portion of the Ras/PKA effects on stationary phase survival are the result of the regulation of autophagy activity by this signaling pathway.
Figures





References
-
- Herman PK. Curr. Opin. Microbiol. 2002;5:602–607. - PubMed
-
- Werner-Washburne M, Braun EL, Crawford ME, Peck VM. Mol. Microbiol. 1996;19:1159–1166. - PubMed
-
- Pardee AB. Science. 1989;246:603–608. - PubMed
-
- Varmus H, Weinberg RA. Genes and the Biology of Cancer. Scientific American Library; New York: 1993.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

