A novel marker of tissue junctions, collagen XXII
- PMID: 15016833
- PMCID: PMC2925840
- DOI: 10.1074/jbc.M400536200
A novel marker of tissue junctions, collagen XXII
Abstract
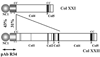
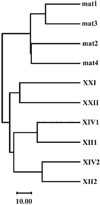
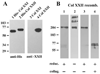
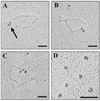
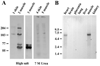
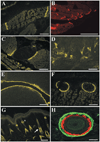
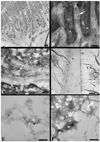
Here we describe a novel specific component of tissue junctions, collagen XXII. It was first identified by screening an EST data base and subsequently expressed as a recombinant protein and characterized as an authentic tissue component. The COL22A1 gene on human chromosome 8q24.2 encodes a collagen that structurally belongs to the FACIT protein family (fibril-associated collagens with interrupted triple helices). Collagen XXII exhibits a striking restricted localization at tissue junctions such as the myotendinous junction in skeletal and heart muscle, the articular cartilage-synovial fluid junction, or the border between the anagen hair follicle and the dermis in the skin. It is deposited in the basement membrane zone of the myotendinous junction and the hair follicle and associated with the extrafibrillar matrix in cartilage. In situ hybridization of myotendinous junctions revealed that muscle cells produce collagen XXII, and functional tests demonstrated that collagen XXII acts as a cell adhesion ligand for skin epithelial cells and fibroblasts. This novel gene product, collagen XXII, is the first specific extracellular matrix protein present only at tissue junctions.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

