Monitoring RNA release from human rhinovirus by dynamic force microscopy
- PMID: 15016841
- PMCID: PMC371065
- DOI: 10.1128/jvi.78.7.3203-3209.2004
Monitoring RNA release from human rhinovirus by dynamic force microscopy
Abstract
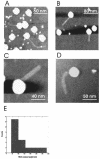
Human rhinoviruses were imaged under physiological conditions by dynamic force microscopy. Topographical images revealed various polygonal areas on the surfaces of the 30-nm viral particles. RNA release was initiated by exposure to a low-pH buffer. The lengths of the RNAs that were released but still connected to the virus capsid varied between 40 and 330 nm, whereas RNA molecules that were completely released from the virus were observed with lengths up to 1 micro m. Fork-like structure elements with 30-nm extensions were sometimes resolved at one end of the RNA molecules. They possibly correspond to the characteristic multi-stem-loop conformation, the internal ribosomal entry site, located at the 5' region of the genome. This study demonstrates that dynamic force microscopy can be used to study viral RNA release in situ under physiological conditions.
Figures



Similar articles
-
The concerted conformational changes during human rhinovirus 2 uncoating.Mol Cell. 2002 Aug;10(2):317-26. doi: 10.1016/s1097-2765(02)00603-2. Mol Cell. 2002. PMID: 12191477
-
ICAM-1 induced rearrangements of capsid and genome prime rhinovirus 14 for activation and uncoating.Proc Natl Acad Sci U S A. 2021 May 11;118(19):e2024251118. doi: 10.1073/pnas.2024251118. Proc Natl Acad Sci U S A. 2021. PMID: 33947819 Free PMC article.
-
Visualization of single receptor molecules bound to human rhinovirus under physiological conditions.Structure. 2005 Sep;13(9):1247-53. doi: 10.1016/j.str.2005.06.012. Structure. 2005. PMID: 16154082
-
The molecular biology of human rhinoviruses.Biochem Soc Symp. 1987;53:63-73. Biochem Soc Symp. 1987. PMID: 2847742 Review.
-
The Dynamic Life of Virus Capsids.Viruses. 2020 Jun 5;12(6):618. doi: 10.3390/v12060618. Viruses. 2020. PMID: 32516952 Free PMC article. Review.
Cited by
-
Analytical Techniques to Characterize the Structure, Properties, and Assembly of Virus Capsids.Anal Chem. 2019 Jan 2;91(1):622-636. doi: 10.1021/acs.analchem.8b04824. Epub 2018 Dec 3. Anal Chem. 2019. PMID: 30383361 Free PMC article. Review.
-
VP4 protein from human rhinovirus 14 is released by pressure and locked in the capsid by the antiviral compound WIN.J Mol Biol. 2007 Feb 9;366(1):295-306. doi: 10.1016/j.jmb.2006.11.033. Epub 2006 Nov 11. J Mol Biol. 2007. PMID: 17161425 Free PMC article.
-
Dynamic force microscopy for imaging of viruses under physiological conditions.Biol Proced Online. 2004;6:120-128. doi: 10.1251/bpo80. Epub 2004 Jun 29. Biol Proced Online. 2004. PMID: 15243650 Free PMC article.
-
Following single antibody binding to purple membranes in real time.EMBO Rep. 2004 Jun;5(6):579-83. doi: 10.1038/sj.embor.7400149. Epub 2004 May 14. EMBO Rep. 2004. PMID: 15143343 Free PMC article.
-
Characterization of early steps in the poliovirus infection process: receptor-decorated liposomes induce conversion of the virus to membrane-anchored entry-intermediate particles.J Virol. 2006 Jan;80(1):172-80. doi: 10.1128/JVI.80.1.172-180.2006. J Virol. 2006. PMID: 16352541 Free PMC article.
References
-
- Binnig, G., C. F. Quate, and C. Gerber. 1986. Atomic force microscope. Phys. Rev. Lett. 56:930-933. - PubMed
-
- Drake, B., C. B. Prater, A. L. Weisenhorn, S. A. Gould, T. R. Albrecht, C. F. Quate, D. S. Cannell, H. G. Hansma, and P. K. Hansma. 1989. Imaging crystals, polymers, and processes in water with the atomic force microscope. Science 243:1586-1589. - PubMed
-
- Drygin, Y. F., O. A. Bordunova, M. O. Gallyamov, and I. V. Yaminsky. 1998. Atomic force microscopy examination of TMV and virion RNA. FEBS Lett. 425:217-221. - PubMed
-
- Han, W., S. M. Lindsay, and T. Jing. 1996. A magnetically driven oscillating probe microscope for operation in liquids. Appl. Phys. Lett. 69:4111-4113.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

