The poliovirus replication machinery can escape inhibition by an antiviral drug that targets a host cell protein
- PMID: 15016860
- PMCID: PMC371039
- DOI: 10.1128/jvi.78.7.3378-3386.2004
The poliovirus replication machinery can escape inhibition by an antiviral drug that targets a host cell protein
Abstract
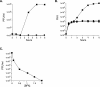
Viral replication depends on specific interactions with host factors. For example, poliovirus RNA replication requires association with intracellular membranes. Brefeldin A (BFA), which induces a major rearrangement of the cellular secretory apparatus, is a potent inhibitor of poliovirus RNA replication. Most aspects governing the relationship between viral replication complex and the host membranes remain poorly defined. To explore these interactions, we used a genetic approach and isolated BFA-resistant poliovirus variants. Mutations within viral proteins 2C and 3A render poliovirus resistant to BFA. In the absence of BFA, viruses containing either or both of these mutations replicated similarly to wild type. In the presence of BFA, viruses carrying a single mutation in 2C or 3A exhibited an intermediate-growth phenotype, while the double mutant was fully resistant. The viral proteins 2C and 3A have critical roles in both RNA replication and vesicle formation. The identification of BFA resistant mutants may facilitate the identification of cellular membrane-associated proteins necessary for induction of vesicle formation and RNA replication. Importantly, our data underscore the dramatic plasticity of the host-virus interactions required for successful viral replication.
Figures



Similar articles
-
Involvement of membrane traffic in the replication of poliovirus genomes: effects of brefeldin A.Virology. 1992 Nov;191(1):166-75. doi: 10.1016/0042-6822(92)90178-r. Virology. 1992. PMID: 1329315
-
Inhibition of poliovirus RNA synthesis by brefeldin A.J Virol. 1992 Apr;66(4):1985-94. doi: 10.1128/JVI.66.4.1985-1994.1992. J Virol. 1992. PMID: 1312615 Free PMC article.
-
A critical role of a cellular membrane traffic protein in poliovirus RNA replication.PLoS Pathog. 2008 Nov;4(11):e1000216. doi: 10.1371/journal.ppat.1000216. Epub 2008 Nov 21. PLoS Pathog. 2008. PMID: 19023417 Free PMC article.
-
Involvement of cellular membrane traffic proteins in poliovirus replication.Cell Cycle. 2007 Jan 1;6(1):36-8. doi: 10.4161/cc.6.1.3683. Epub 2007 Jan 11. Cell Cycle. 2007. PMID: 17245115 Review.
-
IRES-controlled protein synthesis and genome replication of poliovirus.Arch Virol Suppl. 1994;9:279-89. doi: 10.1007/978-3-7091-9326-6_28. Arch Virol Suppl. 1994. PMID: 8032259 Review.
Cited by
-
The Amino Acid Substitution Q65H in the 2C Protein of Swine Vesicular Disease Virus Confers Resistance to Golgi Disrupting Drugs.Front Microbiol. 2016 Apr 27;7:612. doi: 10.3389/fmicb.2016.00612. eCollection 2016. Front Microbiol. 2016. PMID: 27199941 Free PMC article.
-
New small-molecule inhibitors effectively blocking picornavirus replication.J Virol. 2014 Oct;88(19):11091-107. doi: 10.1128/JVI.01877-14. Epub 2014 Jul 9. J Virol. 2014. PMID: 25008939 Free PMC article.
-
Replication and Inhibitors of Enteroviruses and Parechoviruses.Viruses. 2015 Aug 10;7(8):4529-62. doi: 10.3390/v7082832. Viruses. 2015. PMID: 26266417 Free PMC article. Review.
-
Cell-specific establishment of poliovirus resistance to an inhibitor targeting a cellular protein.J Virol. 2015 Apr;89(8):4372-86. doi: 10.1128/JVI.00055-15. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653442 Free PMC article.
-
Wrapping things up about virus RNA replication.Traffic. 2005 Nov;6(11):967-77. doi: 10.1111/j.1600-0854.2005.00339.x. Traffic. 2005. PMID: 16190978 Free PMC article. Review.
References
-
- Beneduce, F., G. Pisani, M. Divizia, A. Pana, and G. Morace. 1995. Complete nucleotide sequence of a cytopathic hepatitis A virus strain isolated in Italy. Virus Res. 36:299-309. - PubMed
-
- Bienz, K., D. Egger, and L. Pasamontes. 1987. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology 160:220-226. - PubMed
-
- Bienz, K., D. Egger, Y. Rasser, and W. Bossart. 1983. Intracellular distribution of poliovirus proteins and the induction of virus-specific cytoplasmic structures. Virology 131:39-48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

