Human immunodeficiency virus type 1 transductive recombination can occur frequently and in proportion to polyadenylation signal readthrough
- PMID: 15016864
- PMCID: PMC371070
- DOI: 10.1128/jvi.78.7.3419-3428.2004
Human immunodeficiency virus type 1 transductive recombination can occur frequently and in proportion to polyadenylation signal readthrough
Abstract
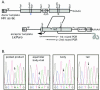
One model for retroviral transduction suggests that template switching between viral RNAs and polyadenylation readthrough sequences is responsible for the generation of acute transforming retroviruses. For this study, we examined reverse transcription products of human immunodeficiency virus (HIV)-based vectors designed to mimic postulated transduction intermediates. For maximization of the discontinuous mode of DNA synthesis proposed to generate transductants, sequences located between the vectors' two long terminal repeats (vector "body" sequences) and polyadenylation readthrough "tail" sequences were made highly homologous. Ten genetic markers were introduced to indicate which products had acquired tail sequences by a process we term transductive recombination. Marker segregation patterns for over 100 individual products were determined, and they revealed that more than half of the progeny proviruses were transductive recombinants. Although most crossovers occurred in regions of homology, about 5% were nonhomologous and some included insertions. Ratios of encapsidated readthrough and polyadenylated transcripts for vectors with wild-type and inactivated polyadenylation signals were compared, and transductive recombination frequencies were found to correlate with the readthrough transcript prevalence. In assays in which either vector body or tail could serve as a recombination donor, recombination between tail and body sequences was at least as frequent as body-body exchange. We propose that transductive recombination may contribute to natural HIV variation by providing a mechanism for the acquisition of nongenomic sequences.
Figures




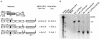
Similar articles
-
Human immunodeficiency virus type 1 genetic recombination is more frequent than that of Moloney murine leukemia virus despite similar template switching rates.J Virol. 2003 Apr;77(8):4577-87. doi: 10.1128/jvi.77.8.4577-4587.2003. J Virol. 2003. PMID: 12663764 Free PMC article.
-
Homologous recombination of copackaged retrovirus RNAs during reverse transcription.J Virol. 1992 Apr;66(4):2378-88. doi: 10.1128/JVI.66.4.2378-2388.1992. J Virol. 1992. PMID: 1372369 Free PMC article.
-
Evidence that retroviral transduction is mediated by DNA not by RNA.Proc Natl Acad Sci U S A. 1990 May;87(9):3604-8. doi: 10.1073/pnas.87.9.3604. Proc Natl Acad Sci U S A. 1990. PMID: 2159155 Free PMC article.
-
Complementarity-directed RNA dimer-linkage promotes retroviral recombination in vivo.Nucleic Acids Res. 2004 Jan 9;32(1):102-14. doi: 10.1093/nar/gkh159. Print 2004. Nucleic Acids Res. 2004. PMID: 14715920 Free PMC article.
-
The remarkable frequency of human immunodeficiency virus type 1 genetic recombination.Microbiol Mol Biol Rev. 2009 Sep;73(3):451-80, Table of Contents. doi: 10.1128/MMBR.00012-09. Microbiol Mol Biol Rev. 2009. PMID: 19721086 Free PMC article. Review.
Cited by
-
Putting an 'End' to HIV mRNAs: capping and polyadenylation as potential therapeutic targets.AIDS Res Ther. 2013 Dec 13;10(1):31. doi: 10.1186/1742-6405-10-31. AIDS Res Ther. 2013. PMID: 24330569 Free PMC article.
-
Hairpin-induced tRNA-mediated (HITME) recombination in HIV-1.Nucleic Acids Res. 2006 May 2;34(8):2206-18. doi: 10.1093/nar/gkl226. Print 2006. Nucleic Acids Res. 2006. PMID: 16670429 Free PMC article.
-
Pseudodiploid genome organization AIDS full-length human immunodeficiency virus type 1 DNA synthesis.J Virol. 2008 Mar;82(5):2376-84. doi: 10.1128/JVI.02100-07. Epub 2007 Dec 19. J Virol. 2008. PMID: 18094172 Free PMC article.
-
Effects of identity minimization on Moloney murine leukemia virus template recognition and frequent tertiary template-directed insertions during nonhomologous recombination.J Virol. 2007 Nov;81(22):12156-68. doi: 10.1128/JVI.01591-07. Epub 2007 Sep 5. J Virol. 2007. PMID: 17804514 Free PMC article.
-
Single point mutations in the zinc finger motifs of the human immunodeficiency virus type 1 nucleocapsid alter RNA binding specificities of the gag protein and enhance packaging and infectivity.J Virol. 2005 Jun;79(12):7756-67. doi: 10.1128/JVI.79.12.7756-7767.2005. J Virol. 2005. PMID: 15919928 Free PMC article.
References
-
- Ashe, M. P., P. Griffin, W. James, and N. J. Proudfoot. 1995. Poly(A) site selection in the HIV-1 provirus: inhibition of promoter-proximal polyadenylation by the downstream major splice donor site. Genes Dev. 9:3008-3025. - PubMed
-
- Besmer, P., J. E. Murphy, P. C. George, F. H. Qiu, P. J. Bergold, L. Lederman, H. W. Snyder, Jr., D. Brodeur, E. E. Zuckerman, and W. D. Hardy. 1986. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature 320:415-421. - PubMed
-
- Bishop, J. M. 1983. Cellular oncogenes and retroviruses. Annu. Rev. Biochem. 52:301-354. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

