Coupling of human immunodeficiency virus type 1 fusion to virion maturation: a novel role of the gp41 cytoplasmic tail
- PMID: 15016865
- PMCID: PMC371074
- DOI: 10.1128/jvi.78.7.3429-3435.2004
Coupling of human immunodeficiency virus type 1 fusion to virion maturation: a novel role of the gp41 cytoplasmic tail
Abstract
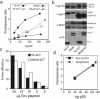
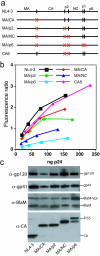
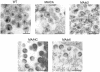
Retrovirus particles are not infectious until they undergo proteolytic maturation to form a functional core. Here we report a link between human immunodeficiency virus type 1 (HIV-1) core maturation and the ability of the virus to fuse with target cells. Using a recently developed reporter assay of HIV-1 virus-cell fusion, we show that immature HIV-1 particles are 5- to 10-fold less active for fusion with target cells than are mature virions. The fusion of mature and immature virions was rendered equivalent by truncating the gp41 cytoplasmic domain or by pseudotyping viruses with the glycoprotein of vesicular stomatitis virus. An analysis of a panel of mutants containing mutated cleavage sites indicated that HIV-1 fusion competence is activated by the cleavage of Gag at any site between the MA and NC segments and not as an indirect consequence of an altered core structure. These results suggest a mechanism by which binding of the gp41 cytoplasmic tail to Gag within immature HIV-1 particles inhibits Env conformational changes on the surface of the virion that are required for membrane fusion. This "inside-out" regulation of HIV-1 fusion could play an important role in the virus life cycle by preventing the entry of immature, noninfectious particles.
Figures
Similar articles
-
A mutation in the human immunodeficiency virus type 1 Gag protein destabilizes the interaction of the envelope protein subunits gp120 and gp41.J Virol. 2006 Mar;80(5):2405-17. doi: 10.1128/JVI.80.5.2405-2417.2006. J Virol. 2006. PMID: 16474147 Free PMC article.
-
Maturation-dependent human immunodeficiency virus type 1 particle fusion requires a carboxyl-terminal region of the gp41 cytoplasmic tail.J Virol. 2007 Sep;81(18):9999-10008. doi: 10.1128/JVI.00592-07. Epub 2007 Jul 3. J Virol. 2007. PMID: 17609279 Free PMC article.
-
Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions.J Virol. 1996 Jan;70(1):341-51. doi: 10.1128/JVI.70.1.341-351.1996. J Virol. 1996. PMID: 8523546 Free PMC article.
-
Exploring HIV-1 Maturation: A New Frontier in Antiviral Development.Viruses. 2024 Sep 6;16(9):1423. doi: 10.3390/v16091423. Viruses. 2024. PMID: 39339899 Free PMC article. Review.
-
The Interplay between HIV-1 Gag Binding to the Plasma Membrane and Env Incorporation.Viruses. 2020 May 16;12(5):548. doi: 10.3390/v12050548. Viruses. 2020. PMID: 32429351 Free PMC article. Review.
Cited by
-
The β-Lactamase Assay: Harnessing a FRET Biosensor to Analyse Viral Fusion Mechanisms.Sensors (Basel). 2016 Jun 23;16(7):950. doi: 10.3390/s16070950. Sensors (Basel). 2016. PMID: 27347948 Free PMC article. Review.
-
Neutralization resistance of virological synapse-mediated HIV-1 Infection is regulated by the gp41 cytoplasmic tail.J Virol. 2012 Jul;86(14):7484-95. doi: 10.1128/JVI.00230-12. Epub 2012 May 2. J Virol. 2012. PMID: 22553332 Free PMC article.
-
A mutation in the human immunodeficiency virus type 1 Gag protein destabilizes the interaction of the envelope protein subunits gp120 and gp41.J Virol. 2006 Mar;80(5):2405-17. doi: 10.1128/JVI.80.5.2405-2417.2006. J Virol. 2006. PMID: 16474147 Free PMC article.
-
Analysis of HIV-1 envelope cytoplasmic tail effects on viral replication.Virology. 2023 Feb;579:54-66. doi: 10.1016/j.virol.2022.12.017. Epub 2023 Jan 2. Virology. 2023. PMID: 36603533 Free PMC article.
-
The HIV-1 Viral Protease Is Activated during Assembly and Budding Prior to Particle Release.J Virol. 2022 May 11;96(9):e0219821. doi: 10.1128/jvi.02198-21. Epub 2022 Apr 19. J Virol. 2022. PMID: 35438536 Free PMC article.
References
-
- Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20:1151-1154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

