The soluble form of human respiratory syncytial virus attachment protein differs from the membrane-bound form in its oligomeric state but is still capable of binding to cell surface proteoglycans
- PMID: 15016875
- PMCID: PMC371076
- DOI: 10.1128/jvi.78.7.3524-3532.2004
The soluble form of human respiratory syncytial virus attachment protein differs from the membrane-bound form in its oligomeric state but is still capable of binding to cell surface proteoglycans
Abstract
The soluble (Gs) and membrane-bound (Gm) forms of human respiratory syncytial virus (HRSV) attachment protein were purified by immunoaffinity chromatography from cultures of HEp-2 cells infected with vaccinia virus recombinants expressing either protein. Sucrose gradient centrifugation indicated that Gs, which is secreted into the culture medium, remains monomeric, whereas Gm is an oligomer, probably a homotetramer. Nevertheless, Gs was capable of binding to the surface of cells in vitro, as assessed by a flow cytometry-based binding assay. The attachment of Gs to cells was inhibited by previous heparinase treatment of living cells, and Gs did not bind to CHO cell mutants defective in proteoglycan biosynthesis. Thus, Gs, as previously reported for the G protein of intact virions, binds to glycosaminoglycans presented at the cell surface as proteoglycans. Deletion of a previously reported heparin binding domain from Gs protein substantially inhibited its ability to bind to cells, but the remaining level of binding was still sensitive to heparinase treatment, suggesting that other regions of the Gs molecule may contribute to attachment to proteoglycans. The significance of these results for HRSV infection is discussed.
Figures
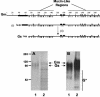
 ), the potential N- (▾) and O-glycosylation sites (|), and the cysteine residues (•). Also indicated are the two mucin-like regions. Formation of the Gs form occurs by translation initiation (i) at Met48 and subsequent cleavage (ii) after residue 65. HRSV Gs (lanes 1) and Gm (lanes 2) proteins were purified by immunoaffinity chromatography, as described in Materials and Methods, and analyzed by Coomassie staining of an SDS-PAGE gel (A) and by Western blotting with monoclonal antibody 63G (B). Molecular weight markers are shown on the left. G* indicates low-molecular-weight bands reacting with antibody 63G.
), the potential N- (▾) and O-glycosylation sites (|), and the cysteine residues (•). Also indicated are the two mucin-like regions. Formation of the Gs form occurs by translation initiation (i) at Met48 and subsequent cleavage (ii) after residue 65. HRSV Gs (lanes 1) and Gm (lanes 2) proteins were purified by immunoaffinity chromatography, as described in Materials and Methods, and analyzed by Coomassie staining of an SDS-PAGE gel (A) and by Western blotting with monoclonal antibody 63G (B). Molecular weight markers are shown on the left. G* indicates low-molecular-weight bands reacting with antibody 63G.



Similar articles
-
Respiratory syncytial virus with the fusion protein as its only viral glycoprotein is less dependent on cellular glycosaminoglycans for attachment than complete virus.Virology. 2002 Mar 15;294(2):296-304. doi: 10.1006/viro.2001.1340. Virology. 2002. PMID: 12009871
-
Iduronic acid-containing glycosaminoglycans on target cells are required for efficient respiratory syncytial virus infection.Virology. 2000 Jun 5;271(2):264-75. doi: 10.1006/viro.2000.0293. Virology. 2000. PMID: 10860881
-
Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells.Arch Virol. 1997;142(6):1247-54. doi: 10.1007/s007050050156. Arch Virol. 1997. PMID: 9229012
-
The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate.J Virol. 2000 Jul;74(14):6442-7. doi: 10.1128/jvi.74.14.6442-6447.2000. J Virol. 2000. PMID: 10864656 Free PMC article.
-
Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G.J Virol. 1999 Aug;73(8):6610-7. doi: 10.1128/JVI.73.8.6610-6617.1999. J Virol. 1999. PMID: 10400758 Free PMC article.
Cited by
-
Effects of Alterations to the CX3C Motif and Secreted Form of Human Respiratory Syncytial Virus (RSV) G Protein on Immune Responses to a Parainfluenza Virus Vector Expressing the RSV G Protein.J Virol. 2019 Mar 21;93(7):e02043-18. doi: 10.1128/JVI.02043-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651356 Free PMC article.
-
Layer-By-Layer Nanoparticle Vaccines Carrying the G Protein CX3C Motif Protect against RSV Infection and Disease.Vaccines (Basel). 2015 Oct 12;3(4):829-49. doi: 10.3390/vaccines3040829. Vaccines (Basel). 2015. PMID: 26473935 Free PMC article.
-
Inhibition of Human Metapneumovirus Binding to Heparan Sulfate Blocks Infection in Human Lung Cells and Airway Tissues.J Virol. 2016 Sep 29;90(20):9237-50. doi: 10.1128/JVI.01362-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27489270 Free PMC article.
-
Identification of linear heparin-binding peptides derived from human respiratory syncytial virus fusion glycoprotein that inhibit infectivity.J Virol. 2007 Jan;81(1):261-71. doi: 10.1128/JVI.01226-06. Epub 2006 Oct 18. J Virol. 2007. PMID: 17050595 Free PMC article.
-
Beyond Infection: The Role of Secreted Viral Proteins in Pathogenesis, Disease Severity and Diagnostic Applications.Cells. 2025 Apr 22;14(9):624. doi: 10.3390/cells14090624. Cells. 2025. PMID: 40358148 Free PMC article. Review.
References
-
- Bembridge, G. P., R. García-Beato, J. A. Lopez, J. A. Melero, and G. Taylor. 1998. Subcellular site of expression and route of vaccination influence pulmonary eosinophilia following respiratory syncytial virus challenge in BALB/c mice sensitized to the attachment G protein. J. Immunol. 161:2473-2480. - PubMed
-
- Blasco, R., and B. Moss. 1995. Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene 158:157-162. - PubMed
-
- Brandenburg, A. H., H. J. Neijens, and A. D. Osterhaus. 2001. Pathogenesis of RSV lower respiratory tract infection: implications for vaccine development. Vaccine 19:2679-2782. - PubMed
-
- Collins, P. L., and G. Mottet. 1992. Oligomerization and post-translational processing of glycoprotein G of human respiratory syncytial virus: altered O-glycosylation in the presence of brefeldin A. J. Gen. Virol. 73:849-863. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

