Differential tissue-specific regulation of antiviral CD8+ T-cell immune responses during chronic viral infection
- PMID: 15016881
- PMCID: PMC371037
- DOI: 10.1128/jvi.78.7.3578-3600.2004
Differential tissue-specific regulation of antiviral CD8+ T-cell immune responses during chronic viral infection
Abstract
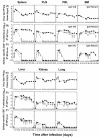
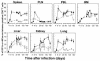
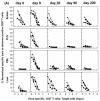
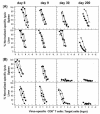
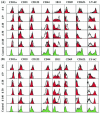
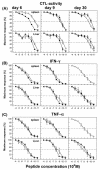
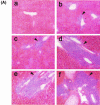
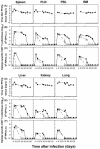
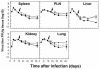
The hallmarks of the immune response to viral infections are the expansion of antigen-specific CD8(+) cytotoxic T lymphocytes (CTLs) after they encounter antigen-presenting cells in the lymphoid tissues and their subsequent redistribution to nonlymphoid tissues to deal with the pathogen. Control mechanisms exist within CTL activation pathways to prevent inappropriate CTL responses against disseminating infections with a broad distribution of pathogen in host tissues. This is demonstrated during overwhelming infection with the noncytolytic murine lymphocytic choriomeningitis virus, in which clonal exhaustion (anergy and/or deletion) of CTLs prevents immune-mediated pathology but allows persistence of the virus. The mechanism by which the immune system determines whether or not to mount a full response to such infections is unknown. Here we present data showing that the initial encounter of specific CTLs with infected cells in lymphoid tissues is critical for this decision. Whether the course of the viral infection is acute or persistent for life primarily depends on the degree and kinetics of CTL exhaustion in infected lymphoid tissues. Virus-driven CTL expansion in lymphoid tissues resulted in the migration of large quantities of CTLs to nonlymphoid tissues, where they persisted at stable levels. Surprisingly, although virus-specific CTLs were rapidly clonally exhausted in lymphoid tissues under conditions of chronic infection, a substantial number of them migrated to nonlymphoid tissues, where they retained an effector phenotype for a long time. However, these cells were unable to control the infection and progressively lost their antiviral capacities (cytotoxicity and cytokine secretion) in a hierarchical manner before their eventual physical elimination. These results illustrate the differential tissue-specific regulation of antiviral T-cell responses during chronic infections and may help us to understand the dynamic relationship between antigen and T-cell populations in many persistent infections in humans.
Figures







Similar articles
-
Control of virus-specific CD8+ T-cell exhaustion and immune-mediated pathology by E3 ubiquitin ligase Cbl-b during chronic viral infection.J Virol. 2008 Apr;82(7):3353-68. doi: 10.1128/JVI.01350-07. Epub 2008 Jan 16. J Virol. 2008. PMID: 18199651 Free PMC article.
-
Impact of CCR7 on priming and distribution of antiviral effector and memory CTL.J Immunol. 2004 Dec 1;173(11):6684-93. doi: 10.4049/jimmunol.173.11.6684. J Immunol. 2004. PMID: 15557160
-
Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone.Nat Immunol. 2008 Jun;9(6):667-75. doi: 10.1038/ni.1605. Epub 2008 Apr 20. Nat Immunol. 2008. PMID: 18425132
-
Immune Exhaustion: Past Lessons and New Insights from Lymphocytic Choriomeningitis Virus.Viruses. 2019 Feb 13;11(2):156. doi: 10.3390/v11020156. Viruses. 2019. PMID: 30781904 Free PMC article. Review.
-
Molecular mechanisms and biological significance of CTL avidity.Curr HIV Res. 2003 Jul;1(3):287-94. doi: 10.2174/1570162033485230. Curr HIV Res. 2003. PMID: 15046253 Review.
Cited by
-
Induction and role of indoleamine 2,3 dioxygenase in mouse models of influenza a virus infection.PLoS One. 2013 Jun 13;8(6):e66546. doi: 10.1371/journal.pone.0066546. Print 2013. PLoS One. 2013. PMID: 23785507 Free PMC article.
-
IL-15 independent maintenance of virus-specific CD8(+) T cells in the CNS during chronic infection.J Neuroimmunol. 2009 Feb 15;207(1-2):32-8. doi: 10.1016/j.jneuroim.2008.11.005. Epub 2008 Dec 23. J Neuroimmunol. 2009. PMID: 19106006 Free PMC article.
-
Active evasion of CTL mediated killing and low quality responding CD8+ T cells contribute to persistence of brucellosis.PLoS One. 2012;7(4):e34925. doi: 10.1371/journal.pone.0034925. Epub 2012 Apr 25. PLoS One. 2012. PMID: 22558103 Free PMC article.
-
Protracted course of lymphocytic choriomeningitis virus WE infection in early life: induction but limited expansion of CD8+ effector T cells and absence of memory CD8+ T cells.J Virol. 2007 Jul;81(14):7338-50. doi: 10.1128/JVI.00062-07. Epub 2007 May 9. J Virol. 2007. PMID: 17494081 Free PMC article.
-
A reservoir of stem-like CD8+ T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response.Sci Immunol. 2021 Oct;6(64):eabg7836. doi: 10.1126/sciimmunol.abg7836. Epub 2021 Sep 2. Sci Immunol. 2021. PMID: 34597124 Free PMC article.
References
-
- Ahmed, R., L. A. Morrison, and D. M. Knipe. 1996. Persistence of viruses, p. 219-249. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, third ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, Pa.
-
- Altman, J. D., P. A. H. Moss, P. J. R. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. - PubMed
-
- Badovinac, V. P., G. A. Corbin, and J. T. Harty. 2000. Off cycling of TNF production by antigen-specific CD8+ T cells is antigen independent. J. Immunol. 165:5387-5391. - PubMed
-
- Battegay, M., S. Cooper, A. Althage, H. Hengartner, and R. M. Zinkernagel. 1991. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24 and 96 well plates. J. Virol. Methods 33:191-198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

