Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression
- PMID: 15016882
- PMCID: PMC371072
- DOI: 10.1128/jvi.78.7.3601-3620.2004
Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression
Abstract
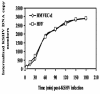
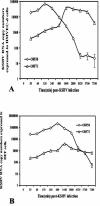
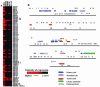
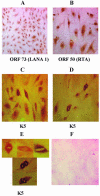
Kaposi's sarcoma-associated herpesvirus (KSHV) infection of in vitro target cells is characterized by the expression of the latency-associated open reading frame (ORF) 73 gene (LANA-1) and the absence of progeny virus production. This default latent infection can be switched into lytic cycle by phorbol ester and by the lytic cycle ORF 50 (RTA) protein. In this study, the kinetics of latent and lytic gene expression immediately following KSHV infection of primary human dermal microvascular endothelial (HMVEC-d) and foreskin fibroblast (HFF) cells were examined by real-time reverse transcriptase PCR and whole-genome array. Within 2 h postinfection (p.i.), high levels of ORF 50 transcripts were detected in both cell types, which declined sharply by 24 h p.i. In contrast, comparatively low levels of ORF 73 expression were detected within 2 h p.i., increased subsequently, were maintained at a steady state, and declined slowly by 120 h p.i. The RTA and LANA-1 proteins were detected in the majority of infected cells by immunoperoxidase assays. In genome array, only 29 of 94 (31%) KSHV genes were expressed, which included 11 immediate-early/early, 8 early, and 5 late lytic genes and 4 latency-associated genes. While the expression of latent ORF 72, 73, and K13 genes continued, nearly all of the lytic genes declined or were undetectable by 8 and 24 h p.i. in HMVEC-d and HFF cells, respectively. Only a limited number of RTA-activated KSHV genes were expressed briefly, and the majority of KSHV genes involved in viral DNA synthesis and structural proteins were not expressed. However, early during infection, the lytic K2, K4, K5, K6, and vIRF2 genes with immune modulation functions and the K7 gene with antiapoptotic function were expressed. Expression of K5 was detected for up to 5 days of observation, and vIRF2 was expressed up to 24 h p.i. The full complement of lytic cycle genes were expressed when 12-O-tetradecanoylphorbol-13-acetate was added to the HMVEC-d cells after 48 h p.i. These data suggest that in contrast to alpha- and betaherpesviruses and some members of gammaherpesviruses, gamma-2 KSHV in vitro infection is characterized by the concurrent expression of latent and a limited number of lytic genes immediately following infection and a subsequent decline and/or absence of lytic gene expression with the persistence of latent genes. Expression of its limited lytic cycle genes could be a "strategy" that evolved in KSHV allowing it to evade the immune system and to provide the necessary factors and time to establish and/or maintain latency during the initial phases of infection. These are unique observations among in vitro herpesvirus infections and may have important implications in KSHV biology and pathogenesis.
Figures




References
-
- Akula, S. M., N. P. Pramod, F.-Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD49c/28) is a cellular receptor for Kaposi's sarcoma associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. - PubMed
-
- Akula, S. M., F.-Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282:245-255. - PubMed
-
- Barnes, B., B. Lubyova, and P. M. Pitha. 2002. On the role of IRF in host defense. J. Interferon Cytokine Res. 22:59-71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

