Movement protein of a closterovirus is a type III integral transmembrane protein localized to the endoplasmic reticulum
- PMID: 15016890
- PMCID: PMC371079
- DOI: 10.1128/jvi.78.7.3704-3709.2004
Movement protein of a closterovirus is a type III integral transmembrane protein localized to the endoplasmic reticulum
Abstract
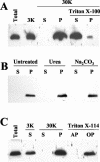
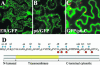
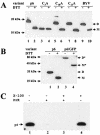
Cell-to-cell movement of beet yellows closterovirus requires four structural proteins and a 6-kDa protein (p6) that is a conventional, nonstructural movement protein. Here we demonstrate that either virus infection or p6 overexpression results in association of p6 with the rough endoplasmic reticulum. The p6 protein possesses a single-span, transmembrane, N-terminal domain and a hydrophilic, C-terminal domain that is localized on the cytoplasmic face of the endoplasmic reticulum. In the infected cells, p6 forms a disulfide bridge via a cysteine residue located near the protein's N terminus. Mutagenic analyses indicated that each of the p6 domains, as well as protein dimerization, is essential for p6 function in virus movement.
Figures

References
-
- Alzhanova, D. V., Y. Hagiwara, V. V. Peremyslov, and V. V. Dolja. 2000. Genetic analysis of the cell-to-cell movement of beet yellows closterovirus. Virology 268:192-200. - PubMed
-
- Beachy, R. N., and M. Heinlein. 2000. Role of p30 in replication and spread of TMV. Traffic 1:540-544. - PubMed
-
- Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256:1604-1607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

