Low pH is required for avian sarcoma and leukosis virus Env-induced hemifusion and fusion pore formation but not for pore growth
- PMID: 15016895
- PMCID: PMC371058
- DOI: 10.1128/jvi.78.7.3753-3762.2004
Low pH is required for avian sarcoma and leukosis virus Env-induced hemifusion and fusion pore formation but not for pore growth
Abstract
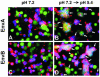
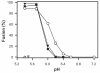
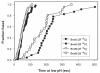
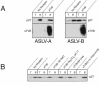
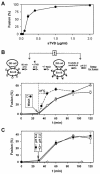
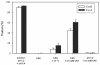
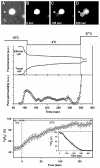
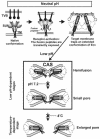
Binding of avian sarcoma and leukosis virus (ASLV) to its cognate receptor on the cell surface causes conformational changes in its envelope protein (Env). It is currently debated whether low pH is required for ASLV infection. To elucidate the role of low pH, we studied the association between ASLV subgroup B (ASLV-B) and liposomes and fusion between effector cells expressing Env from ASLV-A and ASLV-B and target cells expressing cognate receptors. Neither EnvA nor EnvB promoted cell-cell fusion at neutral pH, but lowering the pH resulted in quick and extensive fusion. As expected for a low-pH-triggered reaction, fusion was a steep function of pH. Steps that required low pH were identified. Binding a soluble form of the receptor caused ASLV-B to hydrophobically associate with liposome membranes at neutral pH, indicating that low pH is not required for insertion of Env's fusion peptides into membranes. But both cell-cell hemifusion and fusion pore formation were pH dependent. It is proposed that fusion peptide insertion stabilizes the conformation of ASLV Env into a form that can be acted upon by low pH. At this point, but not before, low pH can induce fusion and is in fact required for fusion to occur. However, low pH is no longer necessary after formation of the initial fusion pore: pore enlargement does not require low pH.
Figures
Similar articles
-
Receptor-induced conformational changes in the SU subunit of the avian sarcoma/leukosis virus A envelope protein: implications for fusion activation.J Virol. 2005 Mar;79(6):3488-99. doi: 10.1128/JVI.79.6.3488-3499.2005. J Virol. 2005. PMID: 15731243 Free PMC article.
-
The avian retrovirus avian sarcoma/leukosis virus subtype A reaches the lipid mixing stage of fusion at neutral pH.J Virol. 2003 Mar;77(5):3058-66. doi: 10.1128/jvi.77.5.3058-3066.2003. J Virol. 2003. PMID: 12584331 Free PMC article.
-
Low pH is required for avian sarcoma and leukosis virus Env-dependent viral penetration into the cytosol and not for viral uncoating.J Virol. 2004 Oct;78(19):10433-41. doi: 10.1128/JVI.78.19.10433-10441.2004. J Virol. 2004. PMID: 15367609 Free PMC article.
-
Alpharetrovirus envelope-receptor interactions.Curr Top Microbiol Immunol. 2003;281:107-36. doi: 10.1007/978-3-642-19012-4_3. Curr Top Microbiol Immunol. 2003. PMID: 12932076 Review.
-
Avian sarcoma and leukosis virus-receptor interactions: from classical genetics to novel insights into virus-cell membrane fusion.Virology. 2006 Jan 5;344(1):25-9. doi: 10.1016/j.virol.2005.09.021. Virology. 2006. PMID: 16364732 Review.
Cited by
-
Receptor-induced conformational changes in the SU subunit of the avian sarcoma/leukosis virus A envelope protein: implications for fusion activation.J Virol. 2005 Mar;79(6):3488-99. doi: 10.1128/JVI.79.6.3488-3499.2005. J Virol. 2005. PMID: 15731243 Free PMC article.
-
Cell-specific targeting of lentiviral vectors mediated by fusion proteins derived from Sindbis virus, vesicular stomatitis virus, or avian sarcoma/leukosis virus.Retrovirology. 2010 Jan 25;7:3. doi: 10.1186/1742-4690-7-3. Retrovirology. 2010. PMID: 20100344 Free PMC article.
-
Hepatitis C virus is primed by CD81 protein for low pH-dependent fusion.J Biol Chem. 2011 Sep 2;286(35):30361-30376. doi: 10.1074/jbc.M111.263350. Epub 2011 Jul 7. J Biol Chem. 2011. PMID: 21737455 Free PMC article.
-
Quantitative imaging of endosome acidification and single retrovirus fusion with distinct pools of early endosomes.Proc Natl Acad Sci U S A. 2012 Oct 23;109(43):17627-32. doi: 10.1073/pnas.1211714109. Epub 2012 Oct 9. Proc Natl Acad Sci U S A. 2012. PMID: 23047692 Free PMC article.
-
The host cell sulfonation pathway contributes to retroviral infection at a step coincident with provirus establishment.PLoS Pathog. 2008 Nov;4(11):e1000207. doi: 10.1371/journal.ppat.1000207. Epub 2008 Nov 14. PLoS Pathog. 2008. PMID: 19008949 Free PMC article.
References
-
- Bates, P., J. A. Young, and H. E. Varmus. 1993. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74:1043-1051. - PubMed
-
- Blumenthal, R., A. Bali-Puri, A. Walter, D. Covell, and O. Eidelman. 1987. pH-dependent fusion of vesicular stomatitis virus with Vero cells. Measurement by dequenching of octadecyl rhodamine fluorescence. J. Biol. Chem. 262:13614-13619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

