The C-terminal transmembrane domain of hepatitis C virus (HCV) RNA polymerase is essential for HCV replication in vivo
- PMID: 15016899
- PMCID: PMC371049
- DOI: 10.1128/jvi.78.7.3797-3802.2004
The C-terminal transmembrane domain of hepatitis C virus (HCV) RNA polymerase is essential for HCV replication in vivo
Abstract
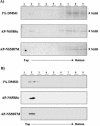
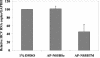
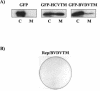
Hepatitis C virus (HCV) RNA replication is dependent on the enzymatic activities of the viral RNA-dependent RNA polymerase NS5B, which is a membrane-anchored protein. Recombinant NS5B lacking the C-terminal transmembrane domain (21 amino acids) is enzymatically active. To address the role of this domain in HCV replication in vivo, we introduced a series of mutations into the NS5B of an HCV subgenomic replicon and examined the replication capabilities of the resultant mutants by a colony formation assay. Replicons lacking the transmembrane domain did not yield any colonies. Furthermore, when Huh-7 cells harboring the HCV subgenomic replicon were treated with a synthetic peptide consisting of the NS5B transmembrane domain fused to the antennapedia peptide, the membrane association of NS5B was completely disrupted. Correspondingly, the HCV RNA titer was reduced by approximately 50%. A scrambled peptide used as a control did not have any effects. These findings suggest that the membrane association of NS5B facilitates HCV RNA synthesis. However, a related transmembrane domain derived from bovine viral diarrhea virus could not replace the HCV NS5B transmembrane segment. This finding suggests that the C-terminal 21 amino acids not only have a membrane-anchoring function but also may perform additional functions for RNA synthesis in vivo.
Figures


Similar articles
-
The C-terminal hydrophobic domain of hepatitis C virus RNA polymerase NS5B can be replaced with a heterologous domain of poliovirus protein 3A.J Virol. 2006 Nov;80(22):11343-54. doi: 10.1128/JVI.02072-05. Epub 2006 Sep 13. J Virol. 2006. PMID: 16971430 Free PMC article.
-
Mutations in NS5B polymerase of hepatitis C virus: impacts on in vitro enzymatic activity and viral RNA replication in the subgenomic replicon cell culture.Virology. 2002 Jun 5;297(2):298-306. doi: 10.1006/viro.2002.1461. Virology. 2002. PMID: 12083828
-
Phosphorylation of hepatitis C virus RNA polymerases ser29 and ser42 by protein kinase C-related kinase 2 regulates viral RNA replication.J Virol. 2014 Oct;88(19):11240-52. doi: 10.1128/JVI.01826-14. Epub 2014 Jul 16. J Virol. 2014. PMID: 25031343 Free PMC article.
-
Hepatitis C virus RNA-dependent RNA polymerase (NS5B polymerase).Curr Top Microbiol Immunol. 2000;242:225-60. doi: 10.1007/978-3-642-59605-6_11. Curr Top Microbiol Immunol. 2000. PMID: 10592663 Review. No abstract available.
-
Using the Hepatitis C Virus RNA-Dependent RNA Polymerase as a Model to Understand Viral Polymerase Structure, Function and Dynamics.Viruses. 2015 Jul 17;7(7):3974-94. doi: 10.3390/v7072808. Viruses. 2015. PMID: 26193306 Free PMC article. Review.
Cited by
-
Activation of anti-hepatitis C virus responses via Toll-like receptor 7.Proc Natl Acad Sci U S A. 2006 Feb 7;103(6):1828-33. doi: 10.1073/pnas.0510801103. Epub 2006 Jan 30. Proc Natl Acad Sci U S A. 2006. PMID: 16446426 Free PMC article.
-
Conserved determinants for membrane association of nonstructural protein 5A from hepatitis C virus and related viruses.J Virol. 2007 Mar;81(6):2745-57. doi: 10.1128/JVI.01279-06. Epub 2006 Dec 27. J Virol. 2007. PMID: 17192310 Free PMC article.
-
Palmitoylation and polymerization of hepatitis C virus NS4B protein.J Virol. 2006 Jun;80(12):6013-23. doi: 10.1128/JVI.00053-06. J Virol. 2006. PMID: 16731940 Free PMC article.
-
Visualisation and analysis of hepatitis C virus non-structural proteins using super-resolution microscopy.Sci Rep. 2018 Sep 11;8(1):13604. doi: 10.1038/s41598-018-31861-0. Sci Rep. 2018. PMID: 30206266 Free PMC article.
-
Annexin A2 is involved in the formation of hepatitis C virus replication complex on the lipid raft.J Virol. 2012 Apr;86(8):4139-50. doi: 10.1128/JVI.06327-11. Epub 2012 Feb 1. J Virol. 2012. PMID: 22301157 Free PMC article.
References
-
- Alter, M. J. 1994. Transmission of hepatitis C virus—route, dose, and titer. N. Engl. J. Med. 330:784-786. - PubMed
-
- Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. - PubMed
-
- Brass, V., E. Bieck, R. Montserret, B. Wolk, J. A. Hellings, H. E. Blum, F. Penin, and D. Moradpour. 2002. An amino-terminal amphipathic alpha-helix mediates membrane association of the hepatitis C virus nonstructural protein 5A. J. Biol. Chem. 277:8130-8139. - PubMed
-
- Derossi, D., S. Calvet, A. Trembleau, A. Brunissen, G. Chassaing, and A. Prochiantz. 1996. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J. Biol. Chem. 271:18188-18193. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

