Ataxin 1, a SCA1 neurodegenerative disorder protein, is functionally linked to the silencing mediator of retinoid and thyroid hormone receptors
- PMID: 15016912
- PMCID: PMC384693
- DOI: 10.1073/pnas.0400615101
Ataxin 1, a SCA1 neurodegenerative disorder protein, is functionally linked to the silencing mediator of retinoid and thyroid hormone receptors
Abstract
Ataxin 1 (Atx1) is a foci-forming polyglutamine protein of unknown function, whose mutant form causes type 1 spinocerebellar ataxia in humans and exerts neurotoxicity in transgenic mouse and fly expressing mutant Atx1. In this study, we demonstrate that Atx1 interacts with the transcriptional corepressor SMRT (silencing mediator of retinoid and thyroid hormone receptors) and with histone deacetylase 3. Atx1 binds chromosomes and mediates transcriptional repression when tethered to DNA. Interaction with SMRT-related factors is a conserved feature of Atx1, because Atx1 also binds SMRTER, a Drosophila cognate of SMRT. Significantly, mutant Atx1 forms aggregates in Drosophila, and such mutant Atx1-mediated aggregates sequester SMRTER. Consistently, the neurodegenerative eye phenotype caused by mutant Atx1 is enhanced by a Smrter mutation and, conversely, is suppressed by a chromosomal duplication that contains the wild type Smrter gene. Together, our results suggest that Atx1 is a transcriptional factor whose mutant form exerts its deleterious effects in part by perturbing corepressor-dependent transcriptional pathways.
Figures

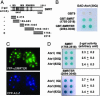
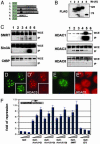

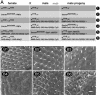
References
-
- Orr, H. T., Chung, M. Y., Banfi, S., Kwiatkowski, T. J., Jr., Servadio, A., Beaudet, A. L., McCall, A. E., Duvick, L. A., Ranum, L. P. & Zoghbi, H. Y. (1993) Nat. Genet. 4, 221–226. - PubMed
-
- Banfi, S., Servadio, A., Chung, M. Y., Kwiatkowski, T. J., Jr., McCall, A. E., Duvick, L. A., Shen, Y., Roth, E. J., Orr, H. T. & Zoghbi, H. Y. (1994) Nat. Genet. 7, 513–520. - PubMed
-
- Zoghbi, H. Y. & Orr, H. T. (2000) Annu. Rev. Neurosci. 23, 217–247. - PubMed
-
- Burright, E. N., Clark, H. B., Servadio, A., Matilla, T., Feddersen, R. M., Yunis, W. S., Duvick, L. A., Zoghbi, H. Y. & Orr, H. T. (1995) Cell 82, 937–948. - PubMed
-
- Fernandez-Funez, P., Nino-Rosales, M. L., de Gouyon, B., She, W. C., Luchak, J. M., Martinez, P., Turiegano, E., Benito, J., Capovilla, M., Skinner, P. J., et al. (2000) Nature 408, 101–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

