Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol
- PMID: 15016920
- PMCID: PMC384699
- DOI: 10.1073/pnas.0307332101
Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol
Abstract
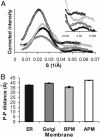
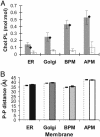
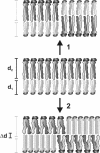
A biological membrane is conceptualized as a system in which membrane proteins are naturally matched to the equilibrium thickness of the lipid bilayer. Cholesterol, in addition to lipid composition, has been suggested to be a major regulator of bilayer thickness in vivo because measurements in vitro have shown that cholesterol can increase the thickness of simple phospholipid/cholesterol bilayers. Using solution x-ray scattering, we have directly measured the average bilayer thickness of exocytic pathway membranes, which contain increasing amounts of cholesterol. The bilayer thickness of membranes of the endoplasmic reticulum, the Golgi, and the basolateral and apical plasma membranes, purified from rat hepatocytes, were determined to be 37.5 +/- 0.4 A, 39.5 +/- 0.4 A, 35.6 +/- 0.6 A, and 42.5 +/- 0.3 A, respectively. After cholesterol depletion using cyclodextrins, Golgi and apical plasma membranes retained their respective bilayer thicknesses whereas the bilayer thickness of the endoplasmic reticulum and the basolateral plasma membrane decreased by 1.0 A. Because cholesterol was shown to have a marginal effect on the thickness of these membranes, we measured whether membrane proteins could modulate thickness. Protein-depleted membranes demonstrated changes in thickness of up to 5 A, suggesting that (i) membrane proteins rather than cholesterol modulate the average bilayer thickness of eukaryotic cell membranes, and (ii) proteins and lipids are not naturally hydrophobically matched in some biological membranes. A marked effect of membrane proteins on the thickness of Escherichia coli cytoplasmic membranes, which do not contain cholesterol, was also observed, emphasizing the generality of our findings.
Figures


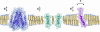
References
-
- Killian, J. A. (1998) Biochim. Biophys. Acta 1376, 401–415. - PubMed
-
- Cornelius, F. (2001) Biochemistry 40, 8842–8851. - PubMed
-
- Johannsson, A., Smith, G. A. & Metcalfe, J. C. (1981) Biochim. Biophys. Acta 641, 416–421. - PubMed
-
- Montecucco, C., Smith, G. A., Dabbeni-sala, F., Johannsson, A., Galante, Y. M. & Bisson, R. (1982) FEBS Lett. 144, 145–148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

