Effect of thyroid hormone binding proteins on insulin receptor binding of B1-thyronine-insulin analogues
- PMID: 15018609
- PMCID: PMC1133761
- DOI: 10.1042/BJ20040177
Effect of thyroid hormone binding proteins on insulin receptor binding of B1-thyronine-insulin analogues
Abstract
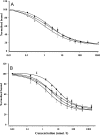
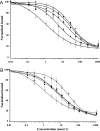
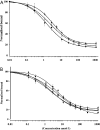
Certain thyronine-insulin analogues, which form non-covalent complexes with plasma proteins, have been shown to act preferentially in the liver. We hypothesized that this property may be dependent on the ability of the analogue to bind to the insulin receptor without prior dissociation from the binding protein. NaB1-L-thyroxyl-insulin, NaB1-3,3',5'-triiodothyronine-insulin, NaB1-D-thyroxyl-insulin and NaB1-L-thyroxyl-aminolauroyl-insulin were compared with insulin for their capacity to inhibit the binding of [125I]TyrA14-insulin to rat liver plasma membrane in albumin-free buffer. Effective doses at 50% maximum inhibition of binding (ED50) were calculated with and without addition of the thyroid hormone binding proteins transthyretin, thyroxine binding globulin and human serum albumin. The binding of thyronine-insulin analogues to insulin receptors was inhibited in a dose-dependent manner by the addition of thyroid hormone binding proteins at concentrations in the physiological range. Complexes of thyronine-insulin analogues with thyroid hormone binding proteins exhibit impaired insulin receptor binding affinities compared with those of the analogues in their free form. Hepatoselectivity in vivo may not depend on binding of the intact complexes to hepatocytes. These results have implications for the physiological role of hormone binding proteins and the in vivo properties of other insulin analogues which bind to plasma proteins.
Figures


Similar articles
-
Novel hepatoselective insulin analogues: studies with covalently linked thyroxyl-insulin complexes.Diabet Med. 1998 Nov;15(11):928-36. doi: 10.1002/(SICI)1096-9136(1998110)15:11<928::AID-DIA702>3.0.CO;2-W. Diabet Med. 1998. PMID: 9827847
-
Comparison of binding of thyroid hormone analogues to hepatic organic anion binding proteins.Biochim Biophys Acta. 1984 Sep 28;801(2):184-8. doi: 10.1016/0304-4165(84)90066-7. Biochim Biophys Acta. 1984. PMID: 6477965
-
Thyroid hormone efflux from monolayer cultures of human fibroblasts and hepatocytes. Effect of lipoproteins and other thyroxine transport proteins.Endocrinology. 1998 Oct;139(10):4311-8. doi: 10.1210/endo.139.10.6231. Endocrinology. 1998. PMID: 9751514
-
Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability.Endocr Rev. 2001 Aug;22(4):451-76. doi: 10.1210/edrv.22.4.0435. Endocr Rev. 2001. PMID: 11493579 Review.
-
Transthyretin as a thyroid hormone carrier: function revisited.Clin Chem Lab Med. 2002 Dec;40(12):1292-300. doi: 10.1515/CCLM.2002.223. Clin Chem Lab Med. 2002. PMID: 12553433 Review.
References
-
- Field J. B. Extraction of insulin by liver. Annu. Rev. Med. 1973;24:309–314. - PubMed
-
- Chap Z., Ishida T., Chou J., Hartley C. J., Entman M. L., Brandenburg D., Jones R. H., Field J. B. First-pass hepatic extraction and metabolic effects of insulin and insulin analogues. Am. J. Physiol. 1987;252:E209–E217. - PubMed
-
- Jones R. H. Insulin action and metabolism. In: Besser G. M., Bodansky H. J., Cudworth A. G., editors. Clinical Diabetes: An Illustrated Text. Raven Press; 1988. pp. 5.1–5.16.
-
- Ruotolo G., Micossi P., Galimberti G., Librenti M. C., Petrella G., Marcovina S., Pozza G., Howard B. V. Effects of intraperitoneal versus subcutaneous insulin administration on lipoprotein metabolism in Type I diabetes. Metabolism. 1990;39:598–604. - PubMed
-
- Olsen C. L., Liu G., Iravani M., Nguyen S., Khourdadjian K., Turner D. S., Waxman K., Selam J. L., Charles M. A. Long-term safety and efficacy of programmable implantable insulin delivery systems. Int. J. Artif. Organs. 1993;16:847–854. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

