C. trachomatis-infection accelerates metabolism of phosphatidylcholine derived from low density lipoprotein but does not affect phosphatidylcholine secretion from hepatocytes
- PMID: 15018642
- PMCID: PMC362871
- DOI: 10.1186/1471-2180-4-8
C. trachomatis-infection accelerates metabolism of phosphatidylcholine derived from low density lipoprotein but does not affect phosphatidylcholine secretion from hepatocytes
Abstract
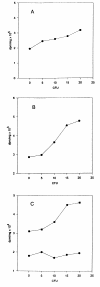
Background: Chlamydia trachomatis is a prevalent sexually transmitted disease and the leading cause of infectious blindness in developing nations. It was not known if C. trachomatis-infection influenced metabolism of lipoprotein-derived phospholipids. Nor was it known if C. trachomatis-infection altered phosphatidylcholine (PC) secretion from hepatocytes. In the current study, low density lipoprotein (LDL)-derived [methyl-3H]PC metabolism was examined in L929 cells infected with C. trachomatis to determine if PC derived from LDL could serve as a potential source of PC trafficked to C. trachomatis. In addition, release of endogenously synthesized [methyl-3H]PC into the medium was examined in rat liver hepatocytes infected with C. trachomatis to determine if infection altered PC secretion.
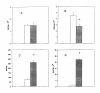
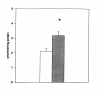
Results: L929 cells 20 h post infection exhibited a 39% (p < 0.05) reduction in radioactivity in PC but total radioactivity incorporation was unaltered compared to controls. Lysophosphatidyl [methyl-3H]choline (LPC) and aqueous [methyl-3H]choline metabolites were elevated 3.6-fold (p < 0.05) and 16.5-fold (p < 0.05), respectively, in C. trachomatis-infected cells and this was due to a 51% increase (p < 0.05) in calcium-dependent phospholipase A2 activity. Hepatocytes 22 h post infection then incubated for 16 h with [methyl-3H]choline showed elevated [methyl-3H]PC biosynthesis but [methyl-3H]PC secreted into the medium was unaltered compared to controls. In contrast, both cellular and medium lyso [methyl-3H]PC were elevated in C. trachomatis-infected cells.
Conclusion: This study is the first to show that metabolism of LDL-derived PC is accelerated in C. trachomatis infection and to support the notion that LDL-delivered PC may potentially serve as a source of PC trafficked to Chlamydia. In addition, C. trachomatis-infection does not inhibit PC secretion from hepatocytes indicating that the pool of newly synthesized PC destined for lipoprotein secretion may differ from the pool of PC used for C. trachomatis membrane biosynthesis.
Figures
Similar articles
-
Specific pools of phospholipids are used for lipoprotein secretion by cultured rat hepatocytes.J Biol Chem. 1986 Apr 5;261(10):4486-91. J Biol Chem. 1986. PMID: 3957904
-
The use of newly synthesized phospholipids for assembly into secreted hepatic lipoproteins.Biochim Biophys Acta. 1989 Nov 6;1006(1):59-69. doi: 10.1016/0005-2760(89)90323-8. Biochim Biophys Acta. 1989. PMID: 2508747
-
Evidence that the rate of phosphatidylcholine catabolism is regulated in cultured rat hepatocytes.Biochim Biophys Acta. 1991 Sep 11;1085(2):167-77. doi: 10.1016/0005-2760(91)90091-u. Biochim Biophys Acta. 1991. PMID: 1892885
-
The role of phosphatidylcholine biosynthesis in the secretion of lipoproteins from hepatocytes.Can J Biochem Cell Biol. 1985 Aug;63(8):870-81. doi: 10.1139/o85-108. Can J Biochem Cell Biol. 1985. PMID: 3904950 Review.
-
Phosphatidylcholine, lysophosphatidylcholine, and lipoprotein secretion by choline-deficient hepatocytes.Nutr Rev. 1990 Jan;48(1):24-6. doi: 10.1111/j.1753-4887.1990.tb02876.x. Nutr Rev. 1990. PMID: 2186314 Review. No abstract available.
Cited by
-
Inhibition of chlamydiae by primary alcohols correlates with the strain-specific complement of plasticity zone phospholipase D genes.Infect Immun. 2006 Jan;74(1):73-80. doi: 10.1128/IAI.74.1.73-80.2006. Infect Immun. 2006. PMID: 16368959 Free PMC article.
-
Killing me softly: chlamydial use of proteolysis for evading host defenses.Trends Microbiol. 2009 Oct;17(10):467-74. doi: 10.1016/j.tim.2009.07.007. Epub 2009 Sep 16. Trends Microbiol. 2009. PMID: 19765998 Free PMC article. Review.
-
Guinea pig genital tract lipidome reveals in vivo and in vitro regulation of phosphatidylcholine 16:0/18:1 and contribution to Chlamydia trachomatis serovar D infectivity.Metabolomics. 2016 Apr;12(4):74. doi: 10.1007/s11306-016-0998-5. Epub 2016 Mar 8. Metabolomics. 2016. PMID: 27642272 Free PMC article.
-
Chlamydia trachomatis growth inhibition and restoration of LDL-receptor level in HepG2 cells treated with mevastatin.Comp Hepatol. 2010 Jan 28;9:3. doi: 10.1186/1476-5926-9-3. Comp Hepatol. 2010. PMID: 20181044 Free PMC article.
References
-
- Vance DE. Phosphatidylcholine metabolism: masochistic enzymology, metabolic regulation, and lipoprotein assembly. Biochem Cell Biol. 1990;68:1151–1165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

