Joint erosion in rheumatoid arthritis: interactions between tumour necrosis factor alpha, interleukin 1, and receptor activator of nuclear factor kappaB ligand (RANKL) regulate osteoclasts
- PMID: 15020327
- PMCID: PMC1754946
- DOI: 10.1136/ard.2003.008458
Joint erosion in rheumatoid arthritis: interactions between tumour necrosis factor alpha, interleukin 1, and receptor activator of nuclear factor kappaB ligand (RANKL) regulate osteoclasts
Abstract
Background: Osteoclasts, specialised bone resorbing cells regulated by RANKL and M-CSF, are implicated in rheumatoid joint erosion. Lymphocyte-monocyte interactions activate bone resorption, this being attributed to tumour necrosis factor alpha (TNFalpha) and interleukin 1 beta (IL1beta) enhanced osteoblast expression of RANKL. In animal studies, TNF potently increases osteoclast formation in the presence of RANKL. RANKL-independent osteoclastogenesis also occurs, though IL1 is required for resorptive function in most studies. These inflammatory cytokines have a pivotal role in rheumatoid arthritis,
Objective: To study the interactions of TNFalpha and IL1beta with RANKL, particularly the time course of the interactions and the role of lymphocytes.
Method: Cultures of lymphocytes and monocytes (osteoclast precursors) or of purified CD14(+) cells alone (osteoclast precursors) were exposed to various combinations of TNFalpha, RANKL, and IL1beta or the inhibitors osteoprotegerin, IL1 receptor antagonist, or neutralising antibodies to RANKL or to IL1. Osteoclastogenesis and resorptive activity were assessed on microscopy of dentine slices.
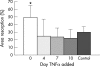
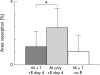
Results: TNFalpha potently increased osteoclast proliferation/differentiation in the presence of RANKL. This effect was greatest when RANKL was present before but not after exposure of osteoclast precursor cells to TNFalpha. The resorptive activity of osteoclasts generated by TNFalpha in the absence of RANKL was critically dependent upon IL1, which was expressed by lymphocyte-monocyte interaction.
Conclusion: TNFalpha potently enhances RANKL mediated osteoclast activity. Interactions between TNFalpha and IL1 also result in osteoclastic activity independently of RANKL. These findings will inform therapeutic approaches to the prevention of joint erosion in rheumatoid arthritis.
Figures
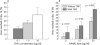


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

