Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome: a phase III trial (E2995)
- PMID: 15020609
- PMCID: PMC5457168
- DOI: 10.1200/JCO.2004.07.048
Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome: a phase III trial (E2995)
Erratum in
- J Clin Oncol. 2004 Jul 1;22(13):2747
Abstract
Purpose: To determine whether adding the multidrug resistance gene-1 (MDR-1) modulator valspodar (PSC 833; Novartis Pharmaceuticals, Hanover, NJ) to chemotherapy provided clinical benefit to patients with poor-risk acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS).
Patients and methods: A phase III randomized study was performed using valspodar plus mitoxantrone, etoposide, and cytarabine (PSC-MEC; n=66) versus MEC (n=63) to treat patients with relapsed or refractory AML and high-risk MDS.
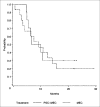
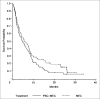
Results: For the PSC-MEC versus MEC arms, complete response (CR) was achieved in 17% versus 25% of patients, respectively (P=not significant). For patients who had not received prior intensive chemotherapy (ie, with secondary AML or high-risk MDS), the CR rate was increased--35% versus 15% for the remaining patients (P=.018); CR rates did not differ between treatment arms. The median disease-free survival in those achieving CR was similar in the two arms (10 versus 9.3 months) as was the patients' overall survival (4.6 versus 5.4 months). The CR rates in MDR+ (69% of patients) versus MDR- patients were similar for those receiving either chemotherapy regimen (16% versus 24%). The CR rate for unfavorable cytogenetic patients (45% of patients) was 13% compared to the remainder, 28% (P=.09). Population pharmacokinetic analysis demonstrated that the clearances of mitoxantrone and etoposide were decreased by 59% and 50%, respectively, supporting the empiric dose reductions in the PSC-MEC arm designed in anticipation of drug interactions between valspodar and the chemotherapeutic agents.
Conclusion: CR rates and overall survival were not improved by using PSC-MEC compared to MEC chemotherapy alone in patients with poor-risk AML or high-risk MDS.
Conflict of interest statement
The authors indicated no potential conflicts of interest.
Figures
References
-
- Velu T, Delbusscher L, Stryckmans P. Daunorubicin in patients with relapsed and refractory acute non-lymphocytic leukemia previously treated with anthracycline. Am J Hematol. 1988;27:224–225. - PubMed
-
- Lowenberg B, Downing J, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. - PubMed
-
- Lepelley P, Soenen V, Preudhomme C, et al. Expression of multidrug resistance p-glycoprotein and its relationship to hematological characteristics and response to treatment in myelodysplastic syndromes. Leukemia. 1994;8:998–1004. - PubMed
-
- List AF. Multidrug resistance: Clinical relevance in acute leukemia. Oncology. 1993;7:23–28. - PubMed
-
- Arceci R. Clinical significance of P-glycoprotein in multidrug resistance malignancies. Blood. 1993;81:2215–2222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA092474/CA/NCI NIH HHS/United States
- U10 CA013650/CA/NCI NIH HHS/United States
- CA66636/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- CA13650/CA/NCI NIH HHS/United States
- U10 CA017145/CA/NCI NIH HHS/United States
- CA11083/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- CA17145/CA/NCI NIH HHS/United States
- M01 RR 00070/RR/NCRR NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- U10 CA180816/CA/NCI NIH HHS/United States
- CA 52168/CA/NCI NIH HHS/United States
- U10 CA011083/CA/NCI NIH HHS/United States
- M01 RR000070/RR/NCRR NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- CA23318/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

