Chemical synthesis and translesion replication of a cis-syn cyclobutane thymine-uracil dimer
- PMID: 15020710
- PMCID: PMC390339
- DOI: 10.1093/nar/gkh342
Chemical synthesis and translesion replication of a cis-syn cyclobutane thymine-uracil dimer
Abstract
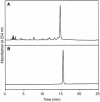
The cytosine base in DNA undergoes hydrolytic deamination at a considerable rate when UV radiation induces formation of a cyclobutane pyrimidine dimer (CPD) with an adjacent pyrimidine base. We have synthesized a phosphoramidite building block of a cis-syn cyclobutane thymine-uracil dimer (T[]U), which is the deaminated form of the CPD at a TC site, and incorporated it into oligodeoxyribonucleotides. The previously reported method for synthesis of the thymine dimer (T[]T) was applied, using partially protected thymidylyl-(3'-5')-2'-deoxyuridine as the starting material, and after triplet- sensitized irradiation, the configuration of the base moiety in the major product was determined by NMR spectroscopy. Presence of the cis-syn cyclobutane dimer in the obtained oligonucleotides was confirmed by UV photoreversal and reaction with T4 endonuclease V. Using a 30mer containing T[]U, translesion synthesis by human DNA polymerase eta was analyzed. There was no difference in the results between the templates containing T[]T and T[]U and pol eta bypassed both lesions with the same efficiency, incorporating two adenylates. This enzyme showed fidelity to base pair formation, but this replication causes a C-->T transition because the original sequence is TC.
Figures
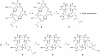
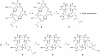




Similar articles
-
Stereoselective formation of a cyclobutane pyrimidine dimer by using N4-acetyl protection of the cytosine base.Nucleic Acids Symp Ser (Oxf). 2008;(52):437-8. doi: 10.1093/nass/nrn222. Nucleic Acids Symp Ser (Oxf). 2008. PMID: 18776441
-
Replication of a cis-syn thymine dimer at atomic resolution.Nature. 2003 Aug 28;424(6952):1083-7. doi: 10.1038/nature01919. Epub 2003 Aug 6. Nature. 2003. PMID: 12904819
-
Accelerated deamination of cytosine residues in UV-induced cyclobutane pyrimidine dimers leads to CC-->TT transitions.Biochemistry. 1996 Aug 6;35(31):10172-81. doi: 10.1021/bi960001x. Biochemistry. 1996. PMID: 8756482
-
[Mechanisms of targeted frameshift mutations--insertion formation under error-prone or SOS synthesis of DNA containing CIS-SYN cyncyclobutane thymine dimers].Mol Biol (Mosk). 2014 Jul-Aug;48(4):531-42. Mol Biol (Mosk). 2014. PMID: 25842840 Review. Russian.
-
A model for targeted substitution mutagenesis during SOS replication of double-stranded DNA containing cis-syn cyclobutane thymine dimers.Environ Mol Mutagen. 2006 Dec;47(9):733-45. doi: 10.1002/em.20256. Environ Mol Mutagen. 2006. PMID: 17111422 Review.
Cited by
-
Photosensitized [2 + 2] cycloaddition of N-acetylated cytosine affords stereoselective formation of cyclobutane pyrimidine dimer.Nucleic Acids Res. 2011 Feb;39(3):1165-75. doi: 10.1093/nar/gkq855. Epub 2010 Sep 29. Nucleic Acids Res. 2011. PMID: 20880992 Free PMC article.
-
A cyclobutane thymine-N4-methylcytosine dimer is resistant to hydrolysis but strongly blocks DNA synthesis.Nucleic Acids Res. 2014 Feb;42(3):2075-84. doi: 10.1093/nar/gkt1039. Epub 2013 Oct 31. Nucleic Acids Res. 2014. PMID: 24185703 Free PMC article.
-
The major mechanism of melanoma mutations is based on deamination of cytosine in pyrimidine dimers as determined by circle damage sequencing.Sci Adv. 2021 Jul 30;7(31):eabi6508. doi: 10.1126/sciadv.abi6508. Print 2021 Jul. Sci Adv. 2021. PMID: 34330711 Free PMC article.
-
Molecular Basis of Substrate Recognition of Endonuclease Q from the Euryarchaeon Pyrococcus furiosus.J Bacteriol. 2020 Jan 2;202(2):e00542-19. doi: 10.1128/JB.00542-19. Print 2020 Jan 2. J Bacteriol. 2020. PMID: 31685534 Free PMC article.
-
Lignin Nanoparticles Deliver Novel Thymine Biomimetic Photo-Adducts with Antimelanoma Activity.Int J Mol Sci. 2022 Jan 14;23(2):915. doi: 10.3390/ijms23020915. Int J Mol Sci. 2022. PMID: 35055101 Free PMC article.
References
-
- Frederico L.A., Kunkel,T.A. and Shaw,B.R. (1990) A sensitive genetic assay for the detection of cytosine deamination: determination of rate constants and the activation energy. Biochemistry, 29, 2532–2537. - PubMed
-
- Liu F.-T. and Yang,N.C. (1978) Photochemistry of cytosine derivatives. 1. Photochemistry of thymidylyl-(3′→5′)-deoxycytidine. Biochemistry, 17, 4865–4876. - PubMed
-
- Douki T. and Cadet,J. (1992) Far-UV photochemistry and photosensitization of 2′-deoxycytidylyl-(3′–5′)-thymidine: isolation and characterization of the main photoproducts. J. Photochem. Photobiol. B., 15, 199–213. - PubMed
-
- Lemaire D.G.E. and Ruzsicska,B.P. (1993) Kinetic analysis of the deamination reactions of cyclobutane dimers of thymidylyl-3′,5′-2′-deoxycytidine and 2′-deoxycytidylyl-3′,5′-thymidine. Biochemistry, 32, 2525–2533. - PubMed
-
- Barak Y., Cohen-Fix,O. and Livneh,Z. (1995) Deamination of cytosine-containing pyrimidine photodimers in UV-irradiated DNA. Significance for UV light mutagenesis. J. Biol. Chem., 270, 24174–24179. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

