Cytoplasmic dynein-dynactin complex is required for spermatid growth but not axoneme assembly in Drosophila
- PMID: 15020714
- PMCID: PMC404038
- DOI: 10.1091/mbc.e03-11-0848
Cytoplasmic dynein-dynactin complex is required for spermatid growth but not axoneme assembly in Drosophila
Abstract
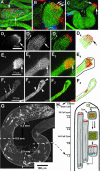
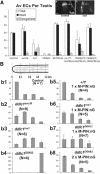
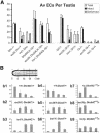
Spermatids derived from a single gonial cell remain interconnected within a cyst and elongate by synchronized growth inside the testis in Drosophila. Cylindrical spectrin-rich elongation cones form at their distal ends during the growth. The mechanism underlying this process is poorly understood. We found that developing sperm tails were abnormally coiled at the growing ends inside the cysts in the Drosophila Dynein light chain 1 (ddlc1) hemizygous mutant testis. A quantitative assay showed that average number of elongation cones was reduced, they were increasingly deformed, and average cyst lengths were shortened in ddlc1 hemizygous testes. These phenotypes were further enhanced by additional partial reduction of Dhc64C and Glued and rescued by Myc-PIN/LC8 expression in the gonial cells in ddlc1 backgrounds. Furthermore, DDLC1, DHC, and GLUED were enriched at the distal ends of growing spermatids. Finally, ultrastructure analysis of ddlc1 testes revealed abnormally formed interspermatid membrane, but the 9 + 2 microtubule organization, the radial spoke structures, and the Dynein arms of the axoneme were normal. Together, these findings suggest that axoneme assembly and spermatid growth involve independent mechanisms in Drosophila and DDLC1 interacts with the Dynein-Dynactin complex at the distal ends of spermatids to maintain the spectrin cytoskeleton assembly and cell growth.
Figures






Similar articles
-
Dynein light chain 1 regulates dynamin-mediated F-actin assembly during sperm individualization in Drosophila.Mol Biol Cell. 2005 Jul;16(7):3107-16. doi: 10.1091/mbc.e05-02-0103. Epub 2005 Apr 13. Mol Biol Cell. 2005. PMID: 15829565 Free PMC article.
-
The Drosophila tctex-1 light chain is dispensable for essential cytoplasmic dynein functions but is required during spermatid differentiation.Mol Biol Cell. 2004 Jul;15(7):3005-14. doi: 10.1091/mbc.e04-01-0013. Epub 2004 Apr 16. Mol Biol Cell. 2004. PMID: 15090621 Free PMC article.
-
Wampa is a dynein subunit required for axonemal assembly and male fertility in Drosophila.Dev Biol. 2020 Jul 15;463(2):158-168. doi: 10.1016/j.ydbio.2020.04.006. Epub 2020 May 6. Dev Biol. 2020. PMID: 32387369 Free PMC article.
-
The Drosophila SUN protein Spag4 cooperates with the coiled-coil protein Yuri Gagarin to maintain association of the basal body and spermatid nucleus.J Cell Sci. 2010 Aug 15;123(Pt 16):2763-72. doi: 10.1242/jcs.066589. Epub 2010 Jul 20. J Cell Sci. 2010. PMID: 20647369 Free PMC article.
-
Structure and molecular basis of spermatid elongation in the Drosophila testis.Open Biol. 2023 Nov;13(11):230136. doi: 10.1098/rsob.230136. Epub 2023 Nov 8. Open Biol. 2023. PMID: 37935354 Free PMC article. Review.
Cited by
-
Dynein light chain 1 (LC8) association enhances microtubule stability and promotes microtubule bundling.J Biol Chem. 2012 Nov 23;287(48):40793-805. doi: 10.1074/jbc.M112.394353. Epub 2012 Oct 4. J Biol Chem. 2012. PMID: 23038268 Free PMC article.
-
The Drosophila RZZ complex - roles in membrane trafficking and cytokinesis.J Cell Sci. 2012 Sep 1;125(Pt 17):4014-25. doi: 10.1242/jcs.099820. Epub 2012 Jun 8. J Cell Sci. 2012. PMID: 22685323 Free PMC article.
-
DNALI1 interacts with the MEIG1/PACRG complex within the manchette and is required for proper sperm flagellum assembly in mice.Elife. 2023 Apr 21;12:e79620. doi: 10.7554/eLife.79620. Elife. 2023. PMID: 37083624 Free PMC article.
-
Identification of genetic networks that act in the somatic cells of the testis to mediate the developmental program of spermatogenesis.PLoS Genet. 2017 Sep 28;13(9):e1007026. doi: 10.1371/journal.pgen.1007026. eCollection 2017 Sep. PLoS Genet. 2017. PMID: 28957323 Free PMC article.
-
Phosphatidylinositol 4,5-bisphosphate directs spermatid cell polarity and exocyst localization in Drosophila.Mol Biol Cell. 2010 May 1;21(9):1546-55. doi: 10.1091/mbc.e09-07-0582. Epub 2010 Mar 17. Mol Biol Cell. 2010. PMID: 20237161 Free PMC article.
References
-
- Abe, T.K., Tanaka, H., Iwanaga, T., Odani, S., and Kuwano, R. (1997). The presence of the 50-kDa subunit of dynactin complex in the nerve growth cone. Biochem. Biophys. Res. Commun. 233, 295–299. - PubMed
-
- Arama, E., Agapite, J., and Steller, H. (2003). Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev. Cell. 4, 687–697. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

