Modulation of microtubule dynamics by tau in living cells: implications for development and neurodegeneration
- PMID: 15020716
- PMCID: PMC420096
- DOI: 10.1091/mbc.e04-01-0062
Modulation of microtubule dynamics by tau in living cells: implications for development and neurodegeneration
Abstract
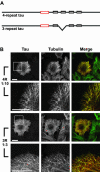
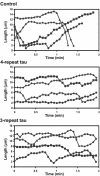
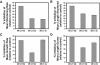
The neural microtubule-associated protein tau binds to and stabilizes microtubules. Because of alternative mRNA splicing, tau is expressed with either 3 or 4 C-terminal repeats. Two observations indicate that differences between these tau isoforms are functionally important. First, the pattern of tau isoform expression is tightly regulated during development. Second, mutation-induced changes in tau RNA splicing cause neuronal cell death and dementia simply by altering the isoform expression ratio. To investigate whether 3- and 4-repeat tau differentially regulate microtubule behavior in cells, we microinjected physiological levels of these two isoforms into EGFP-tubulin-expressing cultured MCF7 cells and measured the effects on the dynamic instability behavior of individual microtubules by time-lapse microscopy. Both isoforms suppressed microtubule dynamics, though to different extents. Specifically, 4-repeat tau reduced the rate and extent of both growing and shortening events. In contrast, 3-repeat tau stabilized most dynamic parameters about threefold less potently than 4-repeat tau and had only a minimal ability to suppress shortening events. These differences provide a mechanistic rationale for the developmental shift in tau isoform expression and are consistent with a loss-of-function model in which abnormal tau isoform expression results in the inability to properly regulate microtubule dynamics, leading to neuronal cell death and dementia.
Figures



References
-
- Bamberger, M.E., and Landreth, G.E. (2002). Inflammation, apoptosis, and Alzheimer's disease. Neuroscientist 8, 276-283. - PubMed
-
- Barghorn, S., and Mandelkow, E. (2002). Toward a unified scheme for the aggregation of tau into Alzheimer paired helical filaments. Biochemistry 41, 14885-14896. - PubMed
-
- Buee, L., Bussiere, T., Buee-Scherrer, V., Delacourte, A., and Hof, P.R. (2000). Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 33, 95-130. - PubMed
-
- Caceres, A., and Kosik, K.S. (1990). Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature 343, 461-463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

