An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal
- PMID: 15024034
- PMCID: PMC2172292
- DOI: 10.1083/jcb.200310055
An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal
Abstract
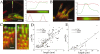
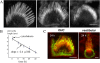
We have previously shown that the seemingly static paracrystalline actin core of hair cell stereocilia undergoes continuous turnover. Here, we used the same approach of transfecting hair cells with actin-green fluorescent protein (GFP) and espin-GFP to characterize the turnover process. Actin and espin are incorporated at the paracrystal tip and flow rearwards at the same rate. The flux rates (approximately 0.002-0.04 actin subunits s(-1)) were proportional to the stereocilia length so that the entire staircase stereocilia bundle was turned over synchronously. Cytochalasin D caused stereocilia to shorten at rates matching paracrystal turnover. Myosins VI and VIIa were localized alongside the actin paracrystal, whereas myosin XVa was observed at the tips at levels proportional to stereocilia lengths. Electron microscopy analysis of the abnormally short stereocilia in the shaker 2 mice did not show the characteristic tip density. We argue that actin renewal in the paracrystal follows a treadmill mechanism, which, together with the myosins, dynamically shapes the functional architecture of the stereocilia bundle.
Figures






References
-
- Anderson, D.W., F.J. Probst, I.A. Belyantseva, R.A. Fridell, L. Beyer, D.M. Martin, D. Wu, B. Kachar, T.B. Friedman, Y. Raphael, and S.A. Camper. 2000. The motor and tail regions of myosin XV are critical for normal structure and function of auditory and vestibular hair cells. Hum. Mol. Genet. 9:1729–1738. - PubMed
-
- Assad, J.A., G.M. Shepherd, and D.P. Corey. 1991. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 7:985–994. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

