Large diameter of palytoxin-induced Na/K pump channels and modulation of palytoxin interaction by Na/K pump ligands
- PMID: 15024043
- PMCID: PMC2217460
- DOI: 10.1085/jgp.200308964
Large diameter of palytoxin-induced Na/K pump channels and modulation of palytoxin interaction by Na/K pump ligands
Abstract
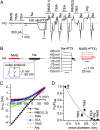
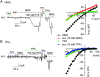
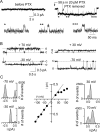
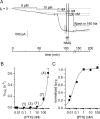
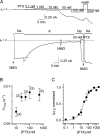
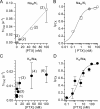
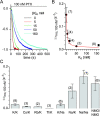
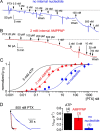
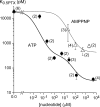
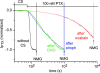
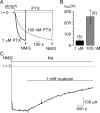
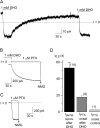
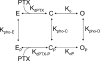
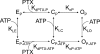
Palytoxin binds to Na/K pumps to generate nonselective cation channels whose pore likely comprises at least part of the pump's ion translocation pathway. We systematically analyzed palytoxin's interactions with native human Na/K pumps in outside-out patches from HEK293 cells over a broad range of ionic and nucleotide conditions, and with or without cardiotonic steroids. With 5 mM internal (pipette) [MgATP], palytoxin activated the conductance with an apparent affinity that was highest for Na(+)-containing (K(+)-free) external and internal solutions, lowest for K(+)-containing (Na(+)-free) external and internal solutions, and intermediate for the mixed external Na(+)/internal K(+), and external K(+)/internal Na(+) conditions; with Na(+) solutions and MgATP, the mean dwell time of palytoxin on the Na/K pump was about one day. With Na(+) solutions, the apparent affinity for palytoxin action was low after equilibration of patches with nucleotide-free pipette solution. That apparent affinity was increased in two phases as the equilibrating [MgATP] was raised over the submicromolar, and submillimolar, ranges, but was increased by pipette MgAMPPNP in a single phase, over the submillimolar range; the apparent affinity at saturating [MgAMPPNP] remained approximately 30-fold lower than at saturating [MgATP]. After palytoxin washout, the conductance decay that reflects palytoxin unbinding was accelerated by cardiotonic steroid. When Na/K pumps were preincubated with cardiotonic steroid, subsequent activation of palytoxin-induced conductance was greatly slowed, even after washout of the cardiotonic steroid, but activation could still be accelerated by increasing palytoxin concentration. These results indicate that palytoxin and a cardiotonic steroid can simultaneously occupy the same Na/K pump, each destabilizing the other. The palytoxin-induced channels were permeable to several large organic cations, including N-methyl-d-glucamine(+), suggesting that the narrowest section of the pore must be approximately 7.5 A wide. Enhanced understanding of palytoxin action now allows its use for examining the structures and mechanisms of the gates that occlude/deocclude transported ions during the normal Na/K pump cycle.
Figures
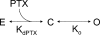
References
-
- Artigas, P., and D.C. Gadsby. 2003. b. Ion occlusion/deocclusion partial reactions in individual palytoxin-modified Na/K pumps. Ann. NY Acad. Sci. 986:116–126. - PubMed
-
- Ahnert-Hilger, G., G.S. Chhatwal, H.J. Hessler, and E. Habermann. 1982. Changes in erythrocyte permeability due to palytoxin as compared to amphotericin B. Biochim. Biophys. Acta. 688:486–494. - PubMed
-
- Ball, W.J., Jr., C.D. Farr, S. Paula, S.M. Keenan, R.K. Delisle, and W.J. Welsh. 2003. Three-dimensional structure-activity relationship modeling of digoxin inhibition and docking to Na+,K+-ATPase. Ann. NY Acad. Sci. 986:296–297. - PubMed
-
- Beaugé, L.A., and I.M. Glynn. 1979. Occlusion of k ions in the unphosphorylated sodium pump. Nature. 280:510–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

