Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist
- PMID: 15024046
- PMCID: PMC2212719
- DOI: 10.1084/jem.20031454
Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist
Abstract
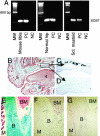
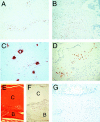
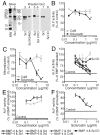
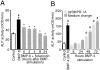
Sclerosteosis, a skeletal disorder characterized by high bone mass due to increased osteoblast activity, is caused by loss of the SOST gene product, sclerostin. The localization in bone and the mechanism of action of sclerostin are not yet known, but it has been hypothesized that it may act as a bone morphogenetic protein (BMP) antagonist. We show here that SOST/sclerostin is expressed exclusively by osteocytes in mouse and human bone and inhibits the differentiation and mineralization of murine preosteoblastic cells (KS483). Although sclerostin shares some of the actions of the BMP antagonist noggin, we show here that it also has actions distinctly different from it. In contrast to noggin, sclerostin did not inhibit basal alkaline phosphatase (ALP) activity in KS483 cells, nor did it antagonize BMP-stimulated ALP activity in mouse C2C12 cells. In addition, sclerostin had no effect on BMP-stimulated Smad phosphorylation and direct transcriptional activation of MSX-2 and BMP response element reporter constructs in KS483 cells. Its unique localization and action on osteoblasts suggest that sclerostin may be the previously proposed osteocyte-derived factor that is transported to osteoblasts at the bone surface and inhibits bone formation.
Figures




References
-
- Truswell, A.S. 1958. Osteopetrosis with syndactyly, a morphologic variant of Albers-Schonberg disease. J. Bone Joint Surg. Br. 40:208–218. - PubMed
-
- Hansen, H. 1967. Sklerosteose. Handbuch der Kinderheilkunde. H. Opitz and F. Schmid, editors. Springer-Verlag, Berlin. 351–355.
-
- Beighton, P., A. Barnard, H. Hamersma, and A. Van Der Wouden. 1984. The syndromic status of sclerosteosis and van Buchem disease. Clin. Genet. 25:175–181. - PubMed
-
- Hamersma, H., J. Gardner, and P. Beighton. 2003. The natural history of sclerosteosis. Clin. Genet. 63:192–197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

