Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes
- PMID: 15024059
- PMCID: PMC371101
- DOI: 10.1128/MCB.24.7.2682-2697.2004
Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes
Abstract
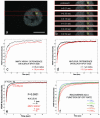
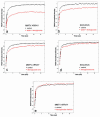
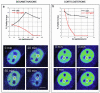
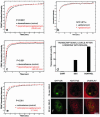
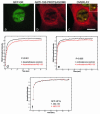
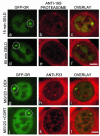
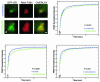
Exchange of the glucocorticoid receptor (GR) at promoter target sites provides the only known system in which transcription factor cycling at a promoter is fast, occurring on a time scale of seconds. The mechanism and function of this rapid exchange are unknown. We provide evidence that proteasome activity is required for rapid GR exchange at a promoter. We also show that chaperones, specifically hsp90, stabilize the binding of GR to the promoter, complicating models in which the associated chaperone, p23, has been proposed to induce GR removal. Our results are the first to connect chaperone and proteasome functions in setting the residence time of a transcription factor at a target promoter. Moreover, our results reveal that longer GR residence times are consistently associated with greater transcriptional output, suggesting a new paradigm in which the rate of rapid exchange provides a means to tune transcriptional levels.
Figures


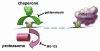
References
-
- Abramowitz, M., and I. A. Stegun. 1970. Handbook of mathematical functions, p. 358-436. Dover Publications, Inc., New York, N.Y.
-
- Bamberger, C. M., M. Wald, A. M. Bamberger, and H. M. Schulte. 1997. Inhibition of mineralocorticoid and glucocorticoid receptor function by the heat shock protein 90-binding agent geldanamycin. Mol. Cell. Endocrinol. 131:233-240. - PubMed
-
- Burakov, D., L. A. Crofts, C. P. Chang, and L. P. Freedman. 2002. Reciprocal recruitment of DRIP/mediator and p160 coactivator complexes in vivo by estrogen receptor. J. Biol. Chem. 277:14359-14362. - PubMed
-
- Connell, P., C. A. Ballinger, J. Jiang, Y. Wu, L. J. Thompson, J. Hohfeld, and C. Patterson. 2001. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat. Cell Biol. 3:93-96. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
