Securin is a target of the UV response pathway in mammalian cells
- PMID: 15024062
- PMCID: PMC371137
- DOI: 10.1128/MCB.24.7.2720-2733.2004
Securin is a target of the UV response pathway in mammalian cells
Abstract
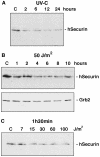
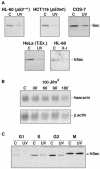
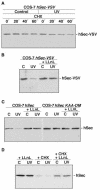
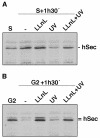
All eukaryotic cells possess elaborate mechanisms to protect genome integrity and ensure survival after DNA damage, ceasing proliferation and granting time for DNA repair. Securin is an inhibitory protein that is bound to a protease called Separase to inhibit sister chromatid separation until the onset of anaphase. At the metaphase-to-anaphase transition, Securin is degraded by the anaphase-promoting complex or cyclosome, and Separase contributes to the release of cohesins from the chromosome, allowing for the segregation of sister chromatids to opposite spindle poles. Here we provide evidence that human Securin (hSecurin) has a novel role in cell cycle arrest after exposure to UV light or ionizing radiation. In fact, irradiation downregulated the level of hSecurin protein, accelerating its degradation via the proteasome and reducing hSecurin mRNA translation, but the presence of hSecurin is necessary for cell proliferation arrest following UV treatment. Moreover, an alteration of UV-induced hSecurin downregulation could lead directly to the accumulation of DNA damage and the subsequent development of malignant tumors.
Figures








Similar articles
-
Protein phosphatase 2A stabilizes human securin, whose phosphorylated forms are degraded via the SCF ubiquitin ligase.Mol Cell Biol. 2006 Jun;26(11):4017-27. doi: 10.1128/MCB.01904-05. Mol Cell Biol. 2006. PMID: 16705156 Free PMC article.
-
UV-induced degradation of securin is mediated by SKP1-CUL1-beta TrCP E3 ubiquitin ligase.J Cell Sci. 2008 Jun 1;121(11):1825-31. doi: 10.1242/jcs.020552. Epub 2008 May 6. J Cell Sci. 2008. PMID: 18460583
-
Separase-mediated cleavage of cohesin at interphase is required for DNA repair.Nature. 2004 Aug 26;430(7003):1044-8. doi: 10.1038/nature02803. Nature. 2004. PMID: 15329725
-
[Activity of separase and its regulation].Yi Chuan. 2004 May;26(3):383-6. Yi Chuan. 2004. PMID: 15640025 Review. Chinese.
-
Cell cycle mechanisms of sister chromatid separation; roles of Cut1/separin and Cut2/securin.Genes Cells. 2000 Jan;5(1):1-8. doi: 10.1046/j.1365-2443.2000.00306.x. Genes Cells. 2000. PMID: 10651900 Review.
Cited by
-
Both p62/SQSTM1-HDAC6-dependent autophagy and the aggresome pathway mediate CDK1 degradation in human breast cancer.Sci Rep. 2017 Aug 30;7(1):10078. doi: 10.1038/s41598-017-10506-8. Sci Rep. 2017. PMID: 28855742 Free PMC article.
-
Cohesin promotes the repair of ionizing radiation-induced DNA double-strand breaks in replicated chromatin.Nucleic Acids Res. 2010 Jan;38(2):477-87. doi: 10.1093/nar/gkp976. Epub 2009 Nov 11. Nucleic Acids Res. 2010. PMID: 19906707 Free PMC article.
-
SCF(FBXW7α) modulates the intra-S-phase DNA-damage checkpoint by regulating Polo like kinase-1 stability.Oncotarget. 2014 Jun 30;5(12):4370-83. doi: 10.18632/oncotarget.2021. Oncotarget. 2014. PMID: 24970797 Free PMC article.
-
Protein phosphatase 2A stabilizes human securin, whose phosphorylated forms are degraded via the SCF ubiquitin ligase.Mol Cell Biol. 2006 Jun;26(11):4017-27. doi: 10.1128/MCB.01904-05. Mol Cell Biol. 2006. PMID: 16705156 Free PMC article.
-
Securin enhances the anti-cancer effects of 6-methoxy-3-(3',4',5'-trimethoxy-benzoyl)-1H-indole (BPR0L075) in human colorectal cancer cells.PLoS One. 2012;7(4):e36006. doi: 10.1371/journal.pone.0036006. Epub 2012 Apr 26. PLoS One. 2012. PMID: 22563433 Free PMC article.
References
-
- Agami, R., and R. Bernards. 2000. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell 102:55-66. - PubMed
-
- Alexandru, G., F. Uhlmann, K. Mechtler, M. A. Poupart, and K. Nasmyth. 2001. Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell 105:459-472. - PubMed
-
- Bartek, J., and J. Lukas. 2001. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr. Opin. Cell Biol. 13:738-747. - PubMed
-
- Bernal, J. A., R. Luna, A. Espina, I. Lazaro, F. Ramos-Morales, F. Romero, C. Arias, A. Silva, M. Tortolero, and J. A. Pintor-Toro. 2002. Human securin interacts with p53 and modulates p53-mediated transcriptional activity and apoptosis. Nat. Genet. 32:306-311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
