Phosphorylation controls Ikaros's ability to negatively regulate the G(1)-S transition
- PMID: 15024069
- PMCID: PMC371126
- DOI: 10.1128/MCB.24.7.2797-2807.2004
Phosphorylation controls Ikaros's ability to negatively regulate the G(1)-S transition
Abstract
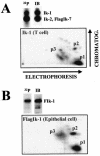
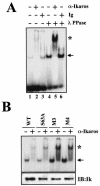
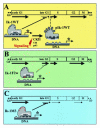
Ikaros is a key regulator of lymphocyte proliferative responses. Inactivating mutations in Ikaros cause antigen-mediated lymphocyte hyperproliferation and the rapid development of leukemia and lymphoma. Here we show that Ikaros's ability to negatively regulate the G(1)-S transition can be modulated by phosphorylation of a serine/threonine-rich conserved region (p1) in exon 8. Ikaros phosphorylation in p1 is induced during the G(1)-S transition. Mutations that prevent phosphorylation in p1 increase Ikaros's ability to impede cell cycle progression and its affinity for DNA. Casein kinase II, whose increased activity in lymphocytes leads to transformation, is a key player in Ikaros p1 phosphorylation. We thus propose that Ikaros's activity as a regulator of the G(1)-S transition is controlled by phosphorylation in response to signaling events that down-modulate its DNA binding activity.
Figures



Similar articles
-
Transcriptional Regulation of PIK3CD and PIKFYVE in T-Cell Acute Lymphoblastic Leukemia by IKAROS and Protein Kinase CK2.Int J Mol Sci. 2021 Jan 15;22(2):819. doi: 10.3390/ijms22020819. Int J Mol Sci. 2021. PMID: 33467550 Free PMC article.
-
Site-Specific Phosphorylation of Ikaros Induced by Low-Dose Ionizing Radiation Regulates Cell Cycle Progression of B Lymphoblast Through CK2 and AKT Activation.Int J Radiat Oncol Biol Phys. 2016 Apr 1;94(5):1207-18. doi: 10.1016/j.ijrobp.2016.01.008. Epub 2016 Jan 14. Int J Radiat Oncol Biol Phys. 2016. PMID: 27026320
-
Restoring Ikaros's wings to solve a leukemia maze.Blood. 2015 Oct 8;126(15):1735-6. doi: 10.1182/blood-2015-08-662544. Blood. 2015. PMID: 26450952
-
Regulation of cellular proliferation in acute lymphoblastic leukemia by Casein Kinase II (CK2) and Ikaros.Adv Biol Regul. 2017 Jan;63:71-80. doi: 10.1016/j.jbior.2016.09.003. Epub 2016 Sep 18. Adv Biol Regul. 2017. PMID: 27666503 Free PMC article. Review.
-
Protein signaling and regulation of gene transcription in leukemia: role of the Casein Kinase II-Ikaros axis.J Investig Med. 2016 Mar;64(3):735-9. doi: 10.1136/jim-2016-000075. Epub 2016 Feb 9. J Investig Med. 2016. PMID: 26912004 Review.
Cited by
-
AF10 plays a key role in the survival of uncommitted hematopoietic cells.PLoS One. 2012;7(12):e51626. doi: 10.1371/journal.pone.0051626. Epub 2012 Dec 19. PLoS One. 2012. PMID: 23284727 Free PMC article.
-
Sumoylation Inhibits the Growth Suppressive Properties of Ikaros.PLoS One. 2016 Jun 17;11(6):e0157767. doi: 10.1371/journal.pone.0157767. eCollection 2016. PLoS One. 2016. PMID: 27315244 Free PMC article.
-
Zinc finger structure-function in Ikaros Marvin A Payne.World J Biol Chem. 2011 Jun 26;2(6):161-6. doi: 10.4331/wjbc.v2.i6.161. World J Biol Chem. 2011. PMID: 21765982 Free PMC article.
-
Regulation of Small GTPase Rab20 by Ikaros in B-Cell Acute Lymphoblastic Leukemia.Int J Mol Sci. 2020 Mar 3;21(5):1718. doi: 10.3390/ijms21051718. Int J Mol Sci. 2020. PMID: 32138279 Free PMC article.
-
Transcriptional Regulation of JARID1B/KDM5B Histone Demethylase by Ikaros, Histone Deacetylase 1 (HDAC1), and Casein Kinase 2 (CK2) in B-cell Acute Lymphoblastic Leukemia.J Biol Chem. 2016 Feb 19;291(8):4004-18. doi: 10.1074/jbc.M115.679332. Epub 2015 Dec 10. J Biol Chem. 2016. PMID: 26655717 Free PMC article.
References
-
- Allman, D., A. Sambandam, S. Kim, J. P. Miller, A. Pagan, D. Well, A. Meraz, and A. Bhandoola. 2003. Thymopoiesis independent of common lymphoid progenitors. Nat. Immunol. 4:168-174. - PubMed
-
- Avitahl, N., S. Winandy, C. Friedrich, B. Jones, Y. Ge, and K. Georgopoulos. 1999. Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity 10:333-343. - PubMed
-
- Boyle, W. J., P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110-149. - PubMed
-
- Channavajhala, P., and D. C. Seldin. 2002. Functional interaction of protein kinase CK2 and c-Myc in lymphomagenesis. Oncogene 21:5280-5288. - PubMed
-
- Classon, M., and E. Harlow. 2002. The retinoblastoma tumour suppressor in development and cancer. Nat. Rev. Cancer 2:910-917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
