The Brn-2 transcription factor links activated BRAF to melanoma proliferation
- PMID: 15024080
- PMCID: PMC371133
- DOI: 10.1128/MCB.24.7.2923-2931.2004
The Brn-2 transcription factor links activated BRAF to melanoma proliferation
Abstract
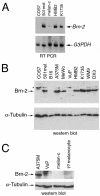
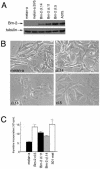
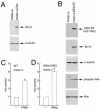
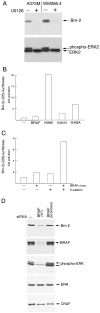
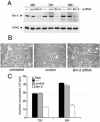
Malignant melanoma, an aggressive and increasingly common cancer, is characterized by a strikingly high rate (70%) of mutations in BRAF, a key component of the mitogen-activated protein (MAP) kinase signaling pathway. How signaling events downstream from BRAF affect the underlying program of gene expression is poorly understood. We show that the Brn-2 POU domain transcription factor is highly expressed in melanoma cell lines but not in melanocytes or melanoblasts and that overexpression of Brn-2 in melanocytes results in increased proliferation. Expression of Brn-2 is strongly upregulated by Ras and MAP kinase signaling. Importantly, the Brn-2 promoter is stimulated by kinase-activating BRAF mutants and endogenous Brn-2 expression is inhibited by RNA interference-mediated downregulation of BRAF. Moreover, silent interfering RNA-mediated depletion of Brn-2 in melanoma cells expressing activated BRAF leads to decreased proliferation. The results suggest that the high levels of Brn-2 expression observed in melanomas link BRAF signaling to increased proliferation.
Figures

References
-
- Angel, P., M. Imagawa, R. Chiu, B. Stein, R. J. Imbra, H. J. Rahmsdorf, C. Jonat, P. Herrlich, and M. Karin. 1987. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell 49:729-739. - PubMed
-
- Bennett, D. C., P. J. Cooper, T. J. Dexter, L. M. Devlin, J. Heasman, and B. Nester. 1989. Cloned mouse melanocyte lines carrying the germline mutations albino and brown: complementation in culture. Development 105:379-385. - PubMed
-
- Bert, A. G., J. Burrows, A. Hawwari, M. A. Vadas, and P. N. Cockerill. 2000. Reconstitution of T cell-specific transcription directed by composite NFAT/Oct elements. J. Immunol. 165:5646-5655. - PubMed
-
- Carreira, S., B. Liu, and C. R. Goding. 2000. The gene encoding the T-box transcription factor Tbx2 is a target for the microphthalmia-associated transcription factor in melanocytes. J. Biol. Chem. 275:21920-21927. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
