Catalytic asymmetric reactions for organic synthesis: the combined C-H activation/Cope rearrangement
- PMID: 15024094
- PMCID: PMC397407
- DOI: 10.1073/pnas.0307556101
Catalytic asymmetric reactions for organic synthesis: the combined C-H activation/Cope rearrangement
Abstract
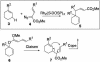
The development of new catalytic asymmetric reactions can lead to exciting new strategies for organic synthesis. This article describes the synthetic utility of the combined C-H activation/Cope rearrangement, achieved by dirhodium tetraprolinate-catalyzed reaction of vinyldiazoacetates with compounds containing allylic C-H bonds. The transformation is highly diastereoselective and enantioselective. The product distribution, however, is highly substrate dependent, the major side products being either direct C-H activation or cyclopropanation.
Figures
References
-
- Jacobsen, E. N., Pfaltz, A. & Yamamoto, H., eds. (1999) Comprehensive Asymmetric Catalysis (Springer, Berlin), Vols. I–III.
-
- Ojima, I. (2000) Catalytic Asymmetric Synthesis (Wiley, New York), 2nd Ed.
-
- Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. eds. (2003) Comprehensive Asymmetric Catalysis (Springer, Berlin), Suppl. 1.
-
- Davies, H. M. L. (1998) Curr. Org. Chem. 2, 463–488.
-
- Davies, H. M. L. & Antoulinakis, E. G. (2001) Org. Reactions 57, 1–326.
LinkOut - more resources
Full Text Sources
Other Literature Sources