A spontaneous, recurrent mutation in divalent metal transporter-1 exposes a calcium entry pathway
- PMID: 15024413
- PMCID: PMC368157
- DOI: 10.1371/journal.pbio.0020050
A spontaneous, recurrent mutation in divalent metal transporter-1 exposes a calcium entry pathway
Abstract
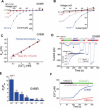
Divalent metal transporter-1 (DMT1/DCT1/Nramp2) is the major Fe(2+) transporter mediating cellular iron uptake in mammals. Phenotypic analyses of animals with spontaneous mutations in DMT1 indicate that it functions at two distinct sites, transporting dietary iron across the apical membrane of intestinal absorptive cells, and transporting endosomal iron released from transferrin into the cytoplasm of erythroid precursors. DMT1 also acts as a proton-dependent transporter for other heavy metal ions including Mn(2+), Co(2+), and Cu(2), but not for Mg(2+) or Ca(2+). A unique mutation in DMT1, G185R, has occurred spontaneously on two occasions in microcytic (mk) mice and once in Belgrade (b) rats. This mutation severely impairs the iron transport capability of DMT1, leading to systemic iron deficiency and anemia. The repeated occurrence of the G185R mutation cannot readily be explained by hypermutability of the gene. Here we show that G185R mutant DMT1 exhibits a new, constitutive Ca(2+) permeability, suggesting a gain of function that contributes to remutation and the mk and b phenotypes.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

