The vaccinia virus-stimulated mitogen-activated protein kinase (MAPK) pathway is required for virus multiplication
- PMID: 15025565
- PMCID: PMC1133850
- DOI: 10.1042/BJ20031375
The vaccinia virus-stimulated mitogen-activated protein kinase (MAPK) pathway is required for virus multiplication
Abstract
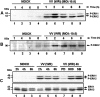
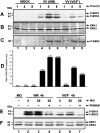
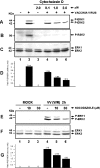
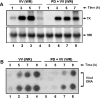
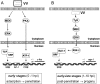
Early events play a decisive role in virus multiplication. We have shown previously that activation of MAPK/ERK1/2 (mitogen-activated protein kinase/extracellular-signal-regulated kinase 1/2) and protein kinase A are pivotal for vaccinia virus (VV) multiplication [de Magalhães, Andrade, Silva, Sousa, Ropert, Ferreira, Kroon, Gazzinelli and Bonjardim (2001) J. Biol. Chem. 276, 38353-38360]. In the present study, we show that VV infection provoked a sustained activation of both ERK1/2 and RSK2 (ribosomal S6 kinase 2). Our results also provide evidence that this pattern of kinase activation depends on virus multiplication and ongoing protein synthesis and is maintained independently of virus DNA synthesis. It is noteworthy that the VGF (VV growth factor), although involved, is not essential for prolonged ERK1/2 activation. Furthermore, our findings suggest that the VV-stimulated ERK1/2 activation also seems to require actin dynamics, microtubule polymerization and tyrosine kinase phosphorylation. The VV-stimulated pathway MEK/ERK1/2/RSK2 (where MEK stands for MAPK/ERK kinase) leads to phosphorylation of the ternary complex factor Elk-1 and expression of the early growth response (egr-1) gene, which kinetically paralleled the kinase activation. The recruitment of this pathway is biologically relevant, since its disruption caused a profound effect on viral thymidine kinase gene expression, viral DNA replication and VV multiplication. This pattern of sustained kinase activation after VV infection is unique. In addition, by connecting upstream signals generated at the cytoskeleton and by tyrosine kinase, the MEK/ERK1/2/RSK2 cascade seems to play a decisive role not only at early stages of the infection, i.e. post-penetration, but is also crucial to define the fate of virus progeny.
Figures


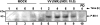


References
-
- Moss B. Poxviridae viruses and their replication. In: Fields B. N., Knipe D. M., Howley P. M., editors. Fields Virology. 3rd edn. vol. 2. Publishers, NY: Lippincott-Raven; 1996. pp. 2637–2672.
-
- Symons J. A., Alcami A., Smith G. L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell (Cambridge, Mass.) 1995;81:551–560. - PubMed
-
- Upton C., Mossman K., McFadden G. Encoding of a homolog of the IFN-γ receptor by myxoma virus. Science. 1992;258:1369–1372. - PubMed
-
- Alcami A. Viral mimicry of cytokines, chemokines and their receptors. Nat. Rev. Immunol. 2003;3:36–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

