Different inhibitory effect of fluvoxamine on omeprazole metabolism between CYP2C19 genotypes
- PMID: 15025747
- PMCID: PMC1884483
- DOI: 10.1111/j.1365-2125.2003.02047.x
Different inhibitory effect of fluvoxamine on omeprazole metabolism between CYP2C19 genotypes
Abstract
Aims: Omeprazole is mainly metabolized by the polymorphic cytochrome P450 (CYP) 2C19. The inhibitory effect of fluvoxamine, an inhibitor of CYP2C19 as well as CYP1A2, on the metabolism of omeprazole was compared between different genotypes for CYP2C19.
Methods: Eighteen volunteers, of whom six were homozygous extensive metabolizers (EMs), six were heterozygous EMs and six were poor metabolizers (PMs) for CYP2C19, participated in the study. A randomized double-blind, placebo-controlled crossover study was performed. All subjects received two six-day courses of either daily 50 mg fluvoxamine or placebo in a randomized fashion with a single oral 40 mg dose of omeprazole on day six in both cases. Plasma concentrations of omeprazole and its metabolites, 5-hydroxyomeprazole, omeprazole sulphone, and fluvoxamine were monitored up to 8 h after the dosing.
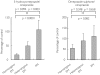
Results: During placebo administration, geometric means of peak concentration (C(max)), under the plasma concentration-time curve from 0 to 8 h (AUC(0,8 h)) and elimination half-life (t(1/2)) of omeprazole were 900 ng ml(-1), 1481 ng ml(-1) h, and 0.6 h in homozygous EMs, 1648 ng ml(-1), 4225 ng ml(-1) h, and 1.1 h in heterozygous EMs, and 2991 ng ml(-1), 11537 ng ml(-1) h, and 2.8 h in PMs, respectively. Fluvoxamine treatment increased C(max) of omeprazole by 3.7-fold (95%CI, 2.4, 5.0-fold, P < 0.01) and 2.0-fold (1.4, 2.6-fold, P < 0.01), AUC(0,8 h) by 6.0-fold (3.3, 8.7-fold, P < 0.001) and 2.4-fold (1.7, 3.2-fold, P < 0.01), AUC(0, infinity ) by 6.2-fold (3.0, 9.3-fold, P < 0.01) and 2.5-fold (1.6, 3.4-fold, P < 0.001) and prolonged t((1/2)) by 2.6-fold (1.9, 3.4-fold, P < 0.001) and 1.4-fold (1.02, 1.7-fold, P < 0.05), respectively. However, no pharmacokinetic parameters were changed in PMs. The AUC(0,8 h) ratios of 5-hydroxyomeprazole to omeprazole were decreased with fluvoxamine in homozygous EMs (P < 0.05) and heterozygous EMs (P < 0.01).
Conclusions: Even a low dose of fluvoxamine increased omeprazole exposure in EMs, but did not increase omeprazole exposure in PMs after a single oral dose of omeprazole. These findings confirm a potent inhibitory effect of fluvoxamine on CYP2C19 activity. The bioavailability of omeprazole might, to some extent, be increased through inhibition of P-glycoprotein during fluvoxamine treatment.
Figures
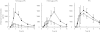
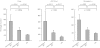
References
-
- Guerreiro AS, Neves BC, Quina MG. Omeprazole in the treatment of peptic ulcers resistant to H2-receptor antagonists. Aliment Pharmacol Ther. 1990;4:309–13. - PubMed
-
- Tybring G, Bottiger Y, Widen J, Bertilsson L. Enantioselective hydroxylation of omeprazole catalyzed by CYP2C19 in Swedish white subjects. Clin Pharmacol Ther. 1997;62:129–37. - PubMed
-
- de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem. 1994;269:15419–22. - PubMed
-
- Bottiger Y, Tybring G, Gotharson E, Bertilsson L. Inhibition of the sulfoxidation of omeprazole by ketoconazole in poor and extensive metabolizers of S-mephenytoin. Clin Pharmacol Ther. 1997;62:384–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

